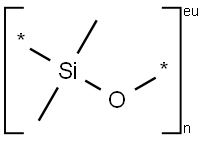
Poly(methylhydrosiloxane)
Synonym(s):Methylhydrogensiloxane polymer;PMHS
- CAS NO.:63148-57-2
- Empirical Formula: C7H22O2Si3
- Molecular Weight: 222.5
- MDL number: MFCD00084478
- EINECS: 613-152-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-26 13:53:03
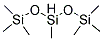
What is Poly(methylhydrosiloxane)?
Description
Poly(methylhydrosiloxane) is a reducing agent. For example, it can be used for the reduction of esters to alcohols as well as the reduction of aldehydes and ketone. It can also be used for the reduction of phosphine oxides to phosphine. Recent study has also used it for conjugate reduction of α, β-Unsaturated Carbonyl and Carboxyl Compounds. Finally, it is also used in greener amine synthesis mediated by reductive amination which is an alternative to borohydrides.
Chemical properties
Colorless liquid
The Uses of Poly(methylhydrosiloxane)
Polymethylhydrosiloxane(PMHS) is an easily handled,inexpensive,non-toxic,and mild reducing agent. PMHS is attractive as a substitute for more expensive or hazardous silanes or siloxanes and as the stoichiometric reductant in catalytic organotin-mediated processes. It can be cross linked by metal catalyst at low temperature to form waterproof membrane. It is used as waterproof agent of textile, glass, pottery, paper, leather, metal, cement and marble etc, especially in textile waterproof. It is used as insulator and cross linker of paper. Poly(methylhydrosiloxane) is used in the reduction of esters to alcohols, catalyzed by a combination of titanocene dichloride and either n-BuLi or EtMgBr. It is a safer alternative to triethoxysilane, B22063, for reduction of phosphine oxides to phosphines, catalyzed by Ti(O-i-Pr)4. It is also used in greener amine synthesis by reductive amination as an alternative to borohydrides.
Preparation
Poly(methylhydrosiloxane) is prepared by hydrolysis of methyldichlorosilane followed by heating (60–150℃) the resultant mixture of cyclic silanes in the presence of hexamethyldisiloxane generates the linear polysiloxane.
General Description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is an environmentally benign reducing agent and has been enhanced for catalytic efficiency. Click here for more information.
Harnessing the reactivity of poly(methylhydrosiloxane) for the reduction and cyclization of biomass to high-value products
Synthesis
Poly(methylhydrosiloxane)(PMHS) is synthesized by the controlled hydrolysis of MeSiHCl2[1]. 
Advantages
PMHS is synthesized by the controlled hydrolysis of MeSiHCl2, a byproduct in producing Me2SiCl2, a feedstock for the silicone industry. As a byproduct of the silicone industry, it is a cheap, easy to handle, and environmentally friendly reducing agent. PMHS is more air and moisture-stable than other silanes and can be stored for long periods of time without loss of activity. In addition to being synthesized from a waste product, PMHS is cheap, stable to air and moisture, and is considered nontoxic[2].
References
[1] HEIN N M, SEO Y, LEE S J, et al. Harnessing the reactivity of poly(methylhydrosiloxane) for the reduction and cyclization of biomass to high-value products?[J]. Green Chemistry, 2019, 10: 2662-2669. DOI:10.1039/C9GC00705A.
[2] Hein, Nicholas M. et al. “Harnessing the reactivity of poly(methylhydrosiloxane) for the reduction and cyclization of biomass to high-value products?.” Green Chemistry 10 (2019): 2662–2669.
Properties of Poly(methylhydrosiloxane)
| Melting point: | <-60°C |
| Boiling point: | 142 °C(lit.) |
| Density | 1.006 g/mL at 25 °C (lit.) |
| vapor pressure | 38 hPa (20 °C) |
| refractive index | n |
| Flash point: | 204 °C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in most ethereal, chlorinated, or hydrocarbon solvents as well as EtOH, i-PrOH, warm DMF, and warm NMP; insoluble in MeOH, DMSO, acetonitrile, and water |
| Specific Gravity | 0.98 |
| PH | 7 (H2O) |
| Water Solubility | Not miscible or difficult to mix in water. |
| CAS DataBase Reference | 63148-57-2 |
| EPA Substance Registry System | Siloxanes and Silicones, Me hydrogen (63148-57-2) |
Safety information for Poly(methylhydrosiloxane)
Computed Descriptors for Poly(methylhydrosiloxane)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Poly(methylhydrosiloxane) CAS 63148-57-2View Details
Poly(methylhydrosiloxane) CAS 63148-57-2View Details
63148-57-2 -
 Poly(methylhydrosiloxane) CAS 63148-57-2View Details
Poly(methylhydrosiloxane) CAS 63148-57-2View Details
63148-57-2 -
 Poly(methyl hydrogen siloxane) CAS 63148-57-2View Details
Poly(methyl hydrogen siloxane) CAS 63148-57-2View Details
63148-57-2 -
 Poly(methylhydrosiloxane) CAS 63148-57-2View Details
Poly(methylhydrosiloxane) CAS 63148-57-2View Details
63148-57-2 -
 Poly(methylhydrosiloxane) CAS 63148-57-2View Details
Poly(methylhydrosiloxane) CAS 63148-57-2View Details
63148-57-2 -
 Poly(methylhydrosiloxane), trimethylsilyl terminated CAS 63148-57-2View Details
Poly(methylhydrosiloxane), trimethylsilyl terminated CAS 63148-57-2View Details
63148-57-2 -
 Methyl Hydrogen Silicone Fluid, Grade: Reagent GradeView Details
Methyl Hydrogen Silicone Fluid, Grade: Reagent GradeView Details
63148-57-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
