Polyethylene glycol mono(2-ethylhexyl) ether
Synonym(s):2-Ethyl hexanol EO-PO nonionic surfactant
- CAS NO.:26468-86-0
- Empirical Formula: C24H50O9
- Molecular Weight: 482.6484
- MDL number: MFCD00804449
- EINECS: 607-943-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52

What is Polyethylene glycol mono(2-ethylhexyl) ether?
Chemical properties
Polyethylene glycol mono(2-ethylhexyl) ether is a low foaming nonionic surfactant with a high cloud point and used as a brightener in acid zinc baths.
The Uses of Polyethylene glycol mono(2-ethylhexyl) ether
Use as wetting agent, permeating agent. For example, used in textile, leather, chemical fiber.
Use as cleansing agent. For example, used in personal care products, household cleaning, industrial cleaning, textile, leather, chemical fiber.
Use as emulsifying agent, dispersing agent.
Use as solubilizing agent.
Side Effects
The main adverse reactions to Polyethylene glycol mono(2-ethylhexyl) ether are: (1) neurotoxin - acute solvent syndrome; (2) occupational hepatotoxicity - secondary hepatotoxicity: cases in which the potential for toxic effects in an occupational setting depends on human ingestion or experimental poisoning in animals.
Synthesis
Preparation of Polyethylene glycol mono(2-ethylhexyl) ether: In a first stage, a 1003g sample of 2-ethylhexanol (EH) was charged to a one-gallon alkoxylation reactor and dehydrated at 115-120°C under a slight nitrogen purge until the moisture content was less than 0.05%. After the dehydrated alcohol was cooled to about 90°C, 3g of boron trifluoride-etherate, included as a catalyst, was charged to the reactor. The alcohol- catalyst mixture was then mixed for 10 minutes, then purged with nitrogen 5 times to remove air and heated to 105°C. Next, 684g of ethylene oxide (EO) was added to the reactor. An exothermic reaction occurred immediately and cooling was applied throughout the EO addition to maintain the reaction temperature within the range of 105-115°C. Following the EO addition, the reaction mixture was digested at 115°C for one hour then purged with nitrogen five times and cooled to 90°C. In a second stage, 31 g of 45% potassium hydroxide, included as a second catalyst, was charged to the reactor. The mixture was then mixed for 10 minutes and then dehydrated at 110°C under nitrogen purge. When the moisture content of the mixture was less than 0.1% (after about 120 minutes of dehydration) 665g of EO was then added to the reactor. Here again, an exothermic reaction occurred and cooling was applied throughout the EO addition to maintain the reaction temperature within the range of 120-130°C. Following the EO addition, the ethoxylation reaction mixture was digested at 125°C-130°C for one hour, then purged with nitrogen five times and cooled to 65°C. Next, 16g of acetic acid was added and then the product mixture was mixed for 15 minutes then discharged. About 2270g of alcohol ethoxylate was collected.
Properties of Polyethylene glycol mono(2-ethylhexyl) ether
| InChI | InChI=1S/C24H50O9/c1-2-3-4-5-6-7-9-26-11-13-28-15-17-30-19-21-32-23-24-33-22-20-31-18-16-29-14-12-27-10-8-25/h25H,2-24H2,1H3 |
| EPA Substance Registry System | Poly(oxy-1,2-ethanediyl), .alpha.-(2-ethylhexyl)-.omega.-hydroxy- (26468-86-0) |
Safety information for Polyethylene glycol mono(2-ethylhexyl) ether
Computed Descriptors for Polyethylene glycol mono(2-ethylhexyl) ether
| InChIKey | YZEARKLIPBHQHG-UHFFFAOYSA-N |
| SMILES | C(OCCCCCCCC)COCCOCCOCCOCCOCCOCCOCCO |
New Products
3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 8-Bromoisoquinoline 2-Bromo-6-flurobenzaldehyde 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Trans-methyl 4-aminocyclohexane- carboxylate HCl N-Boc-L-Alaninol 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE Ibanic Acid Suzetrigine 5,6-Dichloronicotinic acid 2-(2-chloroethoxy)-5-nitrobenzaldehyde Remibrutinib Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Variamine Blue B Diazonium salt Lead II Bromide N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Radiator Flux Zinc Chloride Solution (All Grades) H-Ser(t-Bu)-Ser(t-Bu)-Gly-OHRelated products of tetrahydrofuran


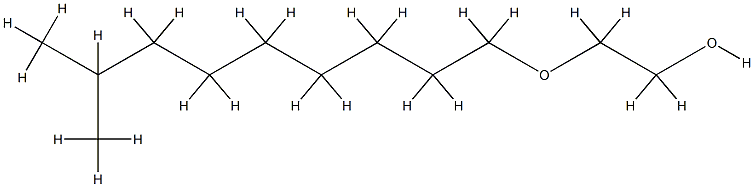



You may like
-
 26468-86-0 Ralufon EN 16-80 99%View Details
26468-86-0 Ralufon EN 16-80 99%View Details
26468-86-0 -
 2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
111771-08-5 -
![3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
2304754-51-4 -
 2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
1073435-67-2 -
 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2477812-43-2 -
 KT-474 98+View Details
KT-474 98+View Details
2432994-31-3 -
 7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 -
 Radiator Flux As requiredView Details
Radiator Flux As requiredView Details
