POLY(ETHYLENE)
- CAS NO.:68037-39-8
- Empirical Formula: C2H4
- Molecular Weight: 0
- MDL number: MFCD00084425
- EINECS: 202-905-8
- Update Date: 2024-12-18 13:37:16
What is POLY(ETHYLENE)?
Chemical properties
light yellow waxy solid
Chemical properties
Before vulcanization, chlorosulphonated polyethylene is a tacky, rubbery
material oflow tensile strength; it is soluble in chlorinated hydrocarbons. The
sulphonyl chloride groups are reactive and may be used for cross-linking the
polymer. Vulcanization is generally carried out by heating with metal oxides
such as litharge or magnesium oxide in the presence of a little water. Reaction
probably proceeds as follows: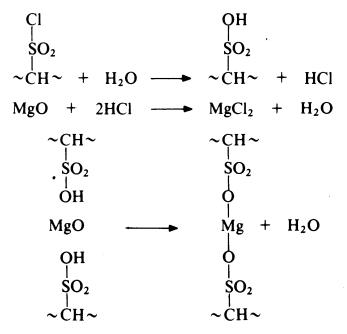
The cross-linked product has good resistance to chemical attack, especially
attack by ozone, oxygen and other oxidizing agents. As a rubber, the material
has excellent mechanical properties and these are maintained over long
periods of use at elevated temperatures. The rather, high cost of the material
limits its use to such applications as sheeting and wire and cable coating
intended for service in demanding conditions.
The Uses of POLY(ETHYLENE)
POLY(ETHYLENE) can be used for rubber hose;electric wire and cable;special synthetic rubber;wires and cables.
Production Methods
This type of elastomer is made by dissolving polyethylene in carbon tetrachloride and then treating it with chlorine and sulfonyl chloride in the presence of a catalyst. After the desired degree of chlorosulfonation has been attained, the residual chlorine–sulfur dioxide mixture is stripped off, a stabilizer is added, and the commercial product is isolated as raw rubber in crumb or film form.
Preparation
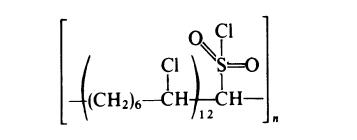
In the preparation of chlorosulphonated polyethylene the polymer (commonly, a low density polyethylene with molecular weight (Mn) of about
20000) is treated with chlorine in the presence of a small amount of sulphur
dioxide. Typically, the reaction is carried out in solution in hot carbon
tetrachloride. Both chloride and sulphonyl chloride groups are introduced
into the polymer, the degree of substitution and the ratio of the two types of
groups depending on the reaction conditions. Commercial products generally
contain about 30% chlorine and 1.5% sulphur, which corresponds to
approximately one chlorine group per 7 carbon atoms and one sulphonyl chloride group per 85 carbon atoms. Such a product may be represented as
follows:
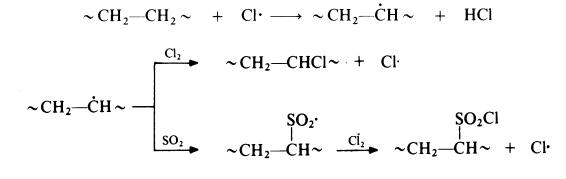
The reaction probably proceeds by the following radical mechanism:
Industrial uses
This material, more commonly known as Hypalon,can be compounded to have an excellentcombination of properties including virtuallytotal resistance to ozone and excellent resistanceto abrasion, weather, heat, flame, oxidizingchemicals, and crack growth. In addition,the material has low moisture absorption, gooddielectric properties, and can be made in a widerange of colors because it does not require carbonblack for reinforcement. Resistance to oilis similar to that of neoprene. Low-temperatureflexibility is fair at –40°C.
The material is made by reacting polyethylenewith chlorine and SO2 to yield chlorosulfonatedpolyethylene. The reaction changes thethermoplastic polyethylene into a synthetic elastomerthat can be compounded and vulcanized.The basic polyethylene contributes chemicalinertness, resistance to damage by moisture, andgood dielectric strength. Inclusion of chlorinein the polymer increases its resistance to flame(makes it self-extinguishing) and contributes toits oil and weather resistance.
Properties of POLY(ETHYLENE)
| Density | 1.28 |
| CAS DataBase Reference | 68037-39-8 |
| EPA Substance Registry System | Ethene, homopolymer, chlorinated, chlorosulfonated (68037-39-8) |
Safety information for POLY(ETHYLENE)
Computed Descriptors for POLY(ETHYLENE)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1