Pneumocandin B0
- CAS NO.:135575-42-7
- Empirical Formula: C50H80N8O17
- Molecular Weight: 1065.21
- MDL number: MFCD28168025
- EINECS: 629-746-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
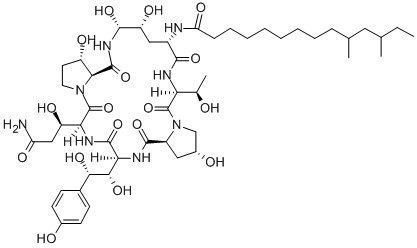
What is Pneumocandin B0?
Description
Pneumocandin B0 is an antifungal lipopeptide that acts by inhibiting the synthesis of β-(1,3)-D-glucan, a component of fungal cell walls (IC50s = 70 and 67 ng/ml for inhibiting glucan synthase in C. albicans and A. fumigatus, respectively). It can be used to synthesize the echinocandin caspofungin acetate .
The Uses of Pneumocandin B0
Pneumocandin B0 is the major analogue of a family of lipopeptides isolated from several species of several different genera, such as Cryptosporiopsis, Glarea and Pezicula. Pneumocandin B0 is a potent antifungal and acts by inhibition of the synthesis of β-(1,3)-D-glucan, an essential component of the cell wall of susceptible fungi.
What are the applications of Application
Pneumocandin B0 is a potent antifungal and inhibitor of β-(1,3)-D-glucan synthesis
Definition
ChEBI: An echinocandin initially isolated as a very minor bioactive fermentation product of Glarea lozoyensis (originally known as Zalerion arboricola). Subsequent random mutagenesis work and optimisation of the fermentation medium permi ted the industrial production of pneumocandin B0, which is used as the starting point for the synthesis of the antifungal drug caspofungin.
Biosynthesis
Pneumocandin B0 (PB0), the precursor of the antifungal drug caspofungin, is a secondary metabolite of the fungus G. lozoyensis, and its biosynthesis is affected by the types and concentrations of nutrients in the medium. Acetyl-CoA and NADPH were identified as the main factors limiting pneumocandin B0 biosynthesis. Other metabolites, such as pyruvate, α-ketoglutaric acid, lactate, unsaturated fatty acids, and previously unreported metabolite γ-aminobutyric acid, were shown to play important roles in pneumocandin B0 biosynthesis and cell growth. The carbon source is a crucial factor affecting cell growth and PB0 production. Transcriptome analysis showed that when G. lozoyensis was cultured with fructose as the carbon source, the expression of genes in the PB0 synthetic gene cluster was downregulated. At the same time, the PB0 yield was increased by 54.76%. When fructose was used as the carbon source, PPP, glycolysis, and branched-chain amino acid metabolism of G. lozoyensis were promoted, which may have provided more NADPH and acetyl-CoA for biosynthesis. Furthermore, the downregulation of the PB0 biosynthesis gene cluster and the TCA cycle resulted in more acetyl-CoA for fatty acid synthesis[3–4].
General Description
Pneumocandin B0, the precursor of the antifungal drug caspofungin, is a lipohexapeptide produced by the fungus Glarea lozoyensis. Oxidative stress and the resulting reactive oxygen species (ROS) are involved in regulating the biosynthesis of it. Acetyl-CoA and NADPH are major factors limiting the biosynthesis of pneumocandin B0. Other metabolites, such as pyruvate, α-ketoglutaric acid, lactate, unsaturated fatty acids, and metabolite γ-aminobutyric acid, also play an important role in the biosynthesis of pneumocandin B0 and cell growth[1-2].
Mechanism of action
Pneumocandin B0 is a potent antifungal and acts by inhibiting the synthesis of β-(1,3)-D-glucan, an essential component of the cell wall of susceptible fungi.
Solubility in organics
Pneumocandin B0 is soluble in ethanol, methanol, DMF and DMSO.
References
[1] Dong Y, et al. Glyap1 regulates pneumocandin B0 synthesis by controlling the intracellular redox balance in Glarea lozoyensis. Applied Microbiology and Biotechnology, 2021; 105: 6707–6718.
[2] Song P, et al. Metabolomics profiling reveals the mechanism of increased pneumocandin B0 production by comparing mutant and parent strains. Journal of Industrial Microbiology Biotechnology, 2018; 45: 767–780.
[3] Ke Zhang. Comparative Transcriptomics Analysis of the Responses of the Filamentous Fungus Glarea lozoyensis to Different Carbon Sources. Frontiers in Microbiology (2020): 190.
[4] Ping Song. Metabolomics profiling reveals the mechanism of increased pneumocandin B0 production by comparing mutant and parent strains. Journal of Industrial Microbiology Biotechnology 45 9 (2018): 767–780.
Properties of Pneumocandin B0
| Melting point: | >230°C (dec.) |
| Boiling point: | 1442.9±65.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 9.86±0.26(Predicted) |
| color | White to Off-White |
| λmax | 276nm(MeOH)(lit.) |
Safety information for Pneumocandin B0
Computed Descriptors for Pneumocandin B0
| InChIKey | DQXPFAADCTZLNL-PYWTXGKPNA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
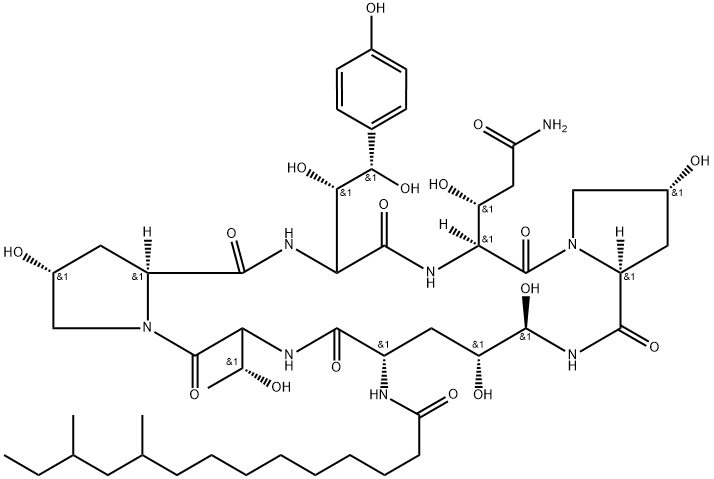
![Pneumocandin A0, 1-[(4R,5S)-5-[(2-aminoethyl)amino]-N2-(10,12-dimethyl-1-oxotetradecyl)-4-hydroxy-L-ornithine]-5-[(3R)-3-hydroxy-L-ornithine]-6-[(4R)-4-hydroxy-L-proline]-](https://img.chemicalbook.in/CAS/20211123/GIF/251095-73-5.gif)

You may like
-
 Pneumocandin B0 CAS 135575-42-7View Details
Pneumocandin B0 CAS 135575-42-7View Details
135575-42-7 -
 Pneumocandin b0 95% CAS 135575-42-7View Details
Pneumocandin b0 95% CAS 135575-42-7View Details
135575-42-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1