Plitidepsin
- CAS NO.:137219-37-5
- Empirical Formula: C57H87N7O15
- Molecular Weight: 1110.34
- MDL number: MFCD00947315
- Update Date: 2024-07-02 08:55:00
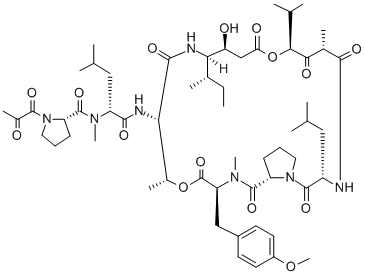
What is Plitidepsin?
Description
Plitidepsin is a cyclic depsipeptide that was first isolated from a Mediterranean marine tunicate (Aplidium albicans) and, at present, is manufactured by total synthesis and commercialized as Aplidin?. Its antitumor activity, observed in preclinical in vitro and in vivo studies has prompted numerous clinical trials to be conducted over the last 17 years, alone or in combination with other anticancer agents.
Definition
ChEBI: Plitidepsin is a didemnin that is didemin B in which the hydroxy group of the 1-(2-hydroxypropanoyl)-L-prolinamide moiety has been oxidised to the corresponding ketone. It was originally isolated from the Mediterranean tunicate Aplidium albicans. It has a role as a marine metabolite, an antineoplastic agent and an anticoronaviral agent.
Biological Activity
Plitidepsin is a cytotoxic peptide originally found in the sea squirt Aplidium albicans. It interacts with a protein (eEF1A2) which is overexpressed in some cancers. This interaction leads to apoptosis. Synthetically produced plitidepsin has been found to have antiproliferative effects on cancer cells. Phase II trials have investigated its activity in tumours such as lung cancer, melanoma and multiple myeloma.
Mechanism of action
Plitidepsin induces apoptosis in multiple myeloma cells, which involves activation of p38 and c-jun NH(2)-terminal kinase signaling, Fas/CD95 translocation to lipid rafts, and ultimately caspase activation. The investigational agent also decreases the proliferation of multiple myeloma cells, an effect mediated by the suppression of several proliferative genes.In a pivotal phase III trial of patients with relapsed or refractory multiple myeloma, plitidepsin in combination with dexamethasone (Dex) significantly reduced the risk of progression or death compared to Dex alone.Plitidepsin has been approved by TGA in Australia for the treatment of multiple myeloma in combination with dexamethasone for patients that relapse after three lines of treatment, including proteasome inhibitors or immunomodulators.
Clinical Use
Plitidepsin is a cyclic depsipeptide isolated from the marine tunicate Aplidium albicans. Plitidepsin displays a broad spectrum of antitumor activities, inducing apoptosis by triggering mitochondrial cytochrome c release, initiating the Fas/ DC95, JNK pathway and activating caspase 3 activation. This agent also inhibits elongation factor 1-a, thereby interfering with protein synthesis, and induces G1 arrest and G2 blockade, thereby inhibiting tumor cell growth.A phase II prospective open-label study (Ferme et al. 2008 ) to evaluate its activity in patients with relapsed/ refractory non-cutaneous PTCL was conducted in 14 patients. They were treated with plitidepsin 3.2 mg/m 2 IV infusion over 1-h on days 1, 8 and 15 every 4 weeks. Eleven patients were evaluable when preliminary results were reported, one was too early and two were non-evaluable. One con fi rmed CR and 3 PR’s were observed with a 36% objective response rate. Median overall survival was 11 months.
Plitidepsin was tolerable in this heavily pretreated population with low hematological toxicity. Transient but reversible ALT elevation was noticed in 7 patients.
Future trials will establish its role in T-cell lymphoma.
Safety Profile
Plitidepsin's safety profile has been extensively studied over both phase I and II/III trials, with the drug presenting mostly transient and tolerated adverse events. Myalgia, alanine aminotransferase/aspartate aminotransferase, creatine phosphokinase increase, fatigue, nausea, and vomiting constituted the most common dose-limiting toxicities in phase I studies. The same adverse events were reported regarding phase II/III studies, along with mild to moderate hematologic abnormalities, such as anaemia and thrombocytopenia. A hypersensitivity reaction was also observed; the event was well-tolerated, except one patient was withdrawn from a trial due to a grade 4 hypersensitivity reaction and hypotension. Notably, plitidepsin with dexamethasone was related to less hepatic enzyme abnormalities compared to plitidepsin alone, while a study assessing the combination of plitidepsin with dexamethasone and bortezomib reported no dose-limiting adverse events[1].
in vitro
In vitro studies have demonstrated that plitidepsin has anti-myeloma activity against 19 multiple myeloma cell lines including cells resistant to anti-myeloma agents frequently used in the clinic (i.e. melphalan, doxorubicin, thalidomide derivatives, and dexamethasone) and primary multiple myeloma cells isolated from patients (13 out 16 showed a response to plitidepsin).
References
[1] Michail Papapanou. “Plitidepsin: Mechanisms and Clinical Profile of a Promising Antiviral Agent against COVID-19.” Journal of Personalized Medicine (2021).
Properties of Plitidepsin
| Melting point: | 152-160° |
| alpha | D -95.9° (c = 1.8 in CHCl3) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| pka | 11.28±0.70(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Plitidepsin
Computed Descriptors for Plitidepsin
| InChIKey | UUSZLLQJYRSZIS-NIORUGPLNA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1