Pivaldehyde
Synonym(s):Pivalaldehyde;Trimethylacetaldehyde
- CAS NO.:630-19-3
- Empirical Formula: C5H10O
- Molecular Weight: 86.13
- MDL number: MFCD00006962
- EINECS: 211-134-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
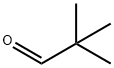
What is Pivaldehyde?
Description
Trimethylacetaldehyde (also known as Pivaldehyde) is the trimethyl form of acetaldehyde. It is an aldehyde with a sterically bulky R group, the tertiary-butyl group being attached to the carbonyl, >C=O. It is a useful reagent in organic synthesis, pharmaceuticals, agrochemicals and dyestuff. It is useful in streoselective synthesis application as well as in aldol condensation reactions. As a derivative of acetaldehyde, trimethyl acetaldehyde can be used for the production of acetate. It can also be used as the precursor for manufacturing of pyridine derivatives, pentaerythritol, and crotonaldehyde.
Chemical properties
Clear colorless liquid
The Uses of Pivaldehyde
Commonly used building block in aldol condensation reactions.
The Uses of Pivaldehyde
Trimethylacetaldehyde is used as a stereoselective synthesis application. It is also used as a building block used in aldol condensation reactions. It is an important raw material and intermediate used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuff.
What are the applications of Application
Pivalaldehyde is a building block used in aldol condensation reactions
Definition
ChEBI: 2,2-dimethylpropanal is a member of the class of propanals that is propanal substituted by two methyl groups at position 2. It is a member of propanals and a 2-methyl-branched fatty aldehyde.
What are the applications of Application
Pivaldehyde is a hazardous by-product released from engine exhaust and is often used as a raw material for organic synthesis or as a reagent in chemical reactions. It can be copolymerised with aldehyde anions, such as acrolein, to prepare unsaturated polyacetals, or a TBPB initiated decarbonylative cascade cyclization of ortho-cyanoarylacrylamides with pivaldehyde leads to tert-butyl containing quinolone-2,4(1H,3H)-diones by formation of both C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds. As well as probing the absolute rate constants of its autoxidation, etc[1-3].
References
Ho, Tse Lok, et al. Pivaldehyde. Fieser and Fieser's Reagents for Organic Synthesis. John Wiley & Sons, Inc. 2006.
https://en.wikipedia.org/wiki/Acetaldehyde#Uses
https://en.wikipedia.org/wiki/Pivaldehyde
https://www.alfa.com/en/catalog/A15013/
References
[1] A. LAPENA; R. S; J L Mateo. Anionic copolymerization of acrolein with aldehydes. III[J]. Journal of Polymer Science: Polymer Symposia, 1973. DOI:10.1002/polc.5070420133.
[2] CUI ZHANG. Decarbonylative cascade cyclization of ortho-cyanoarylacrylamides with pivaldehyde: Access to tert-butyl containing quinolone-2,4(1H,3H)-diones[J]. Tetrahedron, 2022. DOI:10.1016/j.tet.2021.132547.
[3] G. ZAIKOV K. I J Howard. Absolute rate constants for hydrocarbon autoxidation. XIII. Aldehydes: photo-oxidation, co-oxidation, and inhibition[J]. Canadian Journal of Chemistry, 1969. DOI:10.1139/V69-500.
Properties of Pivaldehyde
| Melting point: | 6 °C(lit.) |
| Boiling point: | 74 °C730 mm Hg(lit.) |
| Density | 0.793 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 4 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.793 |
| Water Solubility | Negligible |
| Sensitive | Air Sensitive |
| BRN | 506060 |
| Dielectric constant | 9.0500000000000007 |
| Stability: | Air Sensitive, Volatile |
| CAS DataBase Reference | 630-19-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanal, 2,2-dimethyl-(630-19-3) |
| EPA Substance Registry System | Propanal, 2,2-dimethyl- (630-19-3) |
Safety information for Pivaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pivaldehyde
| InChIKey | FJJYHTVHBVXEEQ-UHFFFAOYSA-N |
Pivaldehyde manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Trimethyl acetaldehyde, 96% CAS 630-19-3View Details
Trimethyl acetaldehyde, 96% CAS 630-19-3View Details
630-19-3 -
 Pivalaldehyde CAS 630-19-3View Details
Pivalaldehyde CAS 630-19-3View Details
630-19-3 -
 Trimethylacetaldehyde 95% CAS 630-19-3View Details
Trimethylacetaldehyde 95% CAS 630-19-3View Details
630-19-3 -
 Trimethylacetaldehyde CAS 630-19-3View Details
Trimethylacetaldehyde CAS 630-19-3View Details
630-19-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1