Pirlimycin
- CAS NO.:79548-73-5
- Empirical Formula: C17H31ClN2O5S
- Molecular Weight: 410.96
- MDL number: MFCD00864968
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
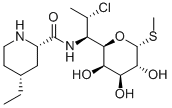
What is Pirlimycin?
The Uses of Pirlimycin
Pirlimycin is a semi-synthetic lincosamide prepared from clindamycin by hydrolysing the propyl N-methylproline and re-annealing a 4-ethylpipecolic acid. Pirlimycin is more hydrophobic than clindamycin and is more potent against a number of important pathogens. Like other members of the lincosamide family, pirlimycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Pirlimycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Pirlimycin has been less extensively researched than the older lincosamides.
Biological Activity
pirlimycin, a lincosamide antibiotic, is effective against gram-positive bacteria, including staphylococcus, bacteroides, streptococcus, and plasmodium. it functions via inhibiting protein synthesis in bacteria by inducing premature dissociation of the peptidyl-trna from the ribosome.
Veterinary Drugs and Treatments
Pirlimycin mastitis tubes are indicated for the treatment of clinical and subclinical mastitis caused by susceptible organisms in lactating dairy cattle.
in vitro
pirlimycin showed activity against helicobacter pylori with a minimal inhibitory concentration [mic] 50 of 4 μg/ml and an mic90 of 64 μg/ml [1].
in vivo
cows were treated with 50 mg of pirlimycin via two intramammary infusions per quarter at a 24-hour interval (2-day) for 2, 5, or 8 days. pirlimycin showed antibiotic therapy against environmental streptococcus spp and staphylococcus aureus intramammary individual and combined infections [1]. specifically, pirlimycin cured environmental streptococcus spp infections in 66.7% (14/21), 85% (17/20), 100% (14/14) in the 2-day group, 5-day group and 8-day group, respectively. s. aureus infections were cured by the treatment of pirlimycin in 13.3% (2/15), 31.3% (5/16), 83.3% (5/6) in the 2-day group, 5-day group and 8-day group, respectively. furthermore, s. aureus, s. dysgalactiae subsp dysgalactiae, and enterococcus spp intramammary infections were eliminated by the extended treatment of pirlimycin in 8-day group [2].
References
[1]. westblom, t., midkiff, b., & czinn, s. in vitro susceptibility ofhelicobacter pylori to trospectomycin, pirlimycin (u-57930e), mirincamycin (u-24729a) and n-demethyl clindamycin (u-26767a). european journal of clinical microbiology & infectious diseases. 1993; 12(7): 560-562.
[2]. b. e. gillespie, h. moorehead, p. lunn, h. h. dowlen, d. l. johnson, k. c. lamar, m. j. lewis, s. j. ivey, j. w. hallberg, s. t. chester, and s. p. oliver. efficacy of extended pirlimycin hydrochloride therapy for treatment of environmental streptococcus spp and staphylococcus aureus intramammary infections in lactating dairy cows. veterinary therapeutics. 2002; 3(4): 373-80.
Properties of Pirlimycin
| Boiling point: | 643.9±55.0 °C(Predicted) |
| Density | 1.31 |
| storage temp. | Store at -20°C |
| solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide |
| form | solid |
| pka | 12.88±0.70(Predicted) |
| Stability: | Hygroscopic |
Safety information for Pirlimycin
Computed Descriptors for Pirlimycin
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4