Pirenzepine hydrochloride
Synonym(s):5,11-Dihydro-11-[(4-methyl-1-piperazinyl)acetyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one dihydrochloride
- CAS NO.:29868-97-1
- Empirical Formula: C19H22ClN5O2
- Molecular Weight: 387.87
- MDL number: MFCD00055214
- EINECS: 249-907-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
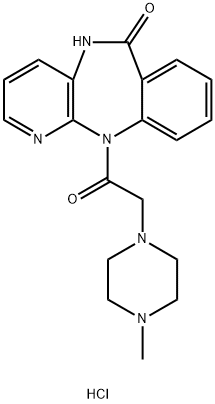
What is Pirenzepine hydrochloride?
Description
Pirenzepine is an antagonist of M1 muscarinic acetylcholine receptors (Ki = 11.48 nM). It is selective for M1 over M2, M3, and M4 receptors (Kis = 602.56, 151.36, and 199.53 nM, respectively). Pirenzepine inhibits ascending reflex contraction of the circular smooth muscle in isolated guinea pig ileal segments induced by intraluminal balloon inflation (IC50 = 501.19 nM). It inhibits methacholine-induced increases in ileal pressure in guinea pigs (ID50 = 724.44 nmol/kg). Pirenzepine inhibits oxotremorine-induced gastric ulcer, gastric acid secretion, and salivation in rats (ED50s = 13, 37.5, and 620 μg/kg i.v., respectively). It prevents form-deprivation myopia (FDM) in a chick model of experimental myopia.
Chemical properties
Solid
Originator
Microsules Bernabo, Microsules Bernabo
The Uses of Pirenzepine hydrochloride
An antiulcerative. Tricyclic gastric-acid inhibitor
The Uses of Pirenzepine hydrochloride
Antiulcerative;M1 antagonist
The Uses of Pirenzepine hydrochloride
An antiulcerative. Tricyclic gastric-acid inhibitor.
What are the applications of Application
Pirenzepine Dihydrochloride is an mAChR M1 selective antagonist
Manufacturing Process
48.4 g of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzo-diazepin-6-one were refluxed in 900 ml of absolute dioxane for 15 minutes. Thereafter, over a period of 45 minutes, 28 ml of chloroacetyl chloride and 52 ml of triethylamine were simultaneously added dropwise to the mixture. The mixture was refluxed for eight hours and then vacuum-filtered after having cooled. The filtrate was evaporated in vacuum. The crystalline residue was recrystallized from acetonitrile in the presence of activated charcoal. MP: 212°-213°C (with decomposition). Yield: 85% of theory.
A mixture of 67.5 g of 11-chloroacetyl-5,11-dihydro-6H-pyrido[2,3b][1,4]benzodiazepin-6-one, 183 ml of N-methylpiperazine and 1.37 liters of absolute benzene was refluxed for 18 hours. Thereafter, the crystalline precipitate was vacuum filtered off, dissolved in aqueous 20% hydrochloric acid, the solution was evaporated in vacuum, the crystalline residue wasdissolved in 250 ml of water while heating, the solution was admixed with 150 ml of isopropanol and active charcoal, filtered, and 2.5 liters of isopropanol were added to the filtrate. After cooling, the precipitate was vacuum filtered off, yielding 70% of theory of the 5,11-dihydro-11-[(4'-methyl-1'-piperazinyl)acetyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one dihydrochloride, M.P. 257259°C (decomp.).
The free base of pirenzepine, obtained from the dihydrochloride by making an aqueous solution thereof alkaline with dilute sodium hydroxide and extracting it with chloroform, had MP: 226°-228°C after recrystallization from methanol/ether.
Therapeutic Function
Antiulcer, Antiemetic
General Description
Pirenzepine dihydrochloride is an anticholinergic agent. It is also considered as an antiulcer drug. It is used to treat myopia, gastric and duodenal ulcers. Pirenzepine dihydrochloride induces the dimerization of muscarinic M1 receptors.
Biochem/physiol Actions
Selective M1 muscarinic acetylcholine receptor antagonist.
storage
Store at RT
Properties of Pirenzepine hydrochloride
| Melting point: | 248-250°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white |
| Water Solubility | Soluble to 100 mM in water |
| Sensitive | Hygroscopic |
| CAS DataBase Reference | 29868-97-1(CAS DataBase Reference) |
Safety information for Pirenzepine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H402:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Pirenzepine hydrochloride
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![Piperidine, 2-methyl-1-[(phenylmethyl)sulfonyl]-](https://img.chemicalbook.in/CAS/20200515/GIF/331847-68-8.gif)


You may like
-
 Pirenzepine Dihydrochloride CAS 29868-97-1View Details
Pirenzepine Dihydrochloride CAS 29868-97-1View Details
29868-97-1 -
 Pirenzepine 2HCl 98% (HPLC) CAS 29868-97-1View Details
Pirenzepine 2HCl 98% (HPLC) CAS 29868-97-1View Details
29868-97-1 -
 Pirenzepine dihydrochloride CAS 29868-97-1View Details
Pirenzepine dihydrochloride CAS 29868-97-1View Details
29868-97-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4