Pipotiazine
- CAS NO.:39860-99-6
- Empirical Formula: C24H33N3O3S2
- Molecular Weight: 475.67
- MDL number: MFCD00941480
- EINECS: 2546596
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
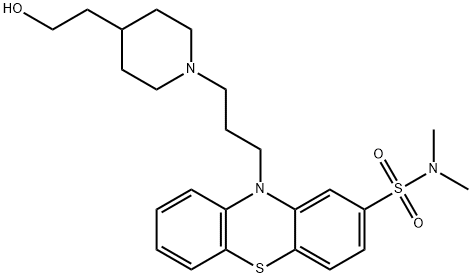
What is Pipotiazine?
Toxicity
Symptoms of overdose include severe extrapyramidal manifestations, hypotension, lethargy and sedation.
Originator
Piportil, Rhone-Poulenc Rorer
The Uses of Pipotiazine
Pipotiazine is drug repurposing for Covid-19: discovery of potential small-molecule inhibitors of spike protein-ACE2 receptor interaction through virtual screening and consensus scoring.
The Uses of Pipotiazine
Antipsychotic.
Indications
For the maintenance treatment of chronic non-agitated schizophrenic patients.
Background
Pipotiazine has actions similar to those of other phenothiazines. Among the different phenothiazine derivatives, it appears to be less sedating and to have a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. However, it produces a high incidence of extra pyramidal reactions. It is used for the maintenance treatment of chronic non-agitated schizophrenic patients. Symptoms of overdose include severe extrapyramidal manifestations, hypotension, lethargy and sedation.
Manufacturing Process
10-(3-Tetrahydropyranyloxypropyl)phenthiazine-2-sulfonic acid dimethylamide prepared by condensing 1-chloro-3-tetrahydropyranyloxy propane with phenthiazine-2-sulfonic acid dimethylamide (melting point 140°C) in xylene in the presence of sodamide.
10-(3-Hydroxypropyl)phenthiazine-2-sulfonic acid dimethylamide was prepared by the action of hydrochloric acid in ethanol on 10-(3tetrahydropyranyloxypropyl)phenthiazine-2-sulfonic acid dimethylamide.
10-(3-Methanesulphonyloxypropyl)phenthiazine-2-sulphonic acid dimethylamide, was obtained by condensing methanesulphonyl chloride in anhydrous pyridine with 10-(3-hydroxypropyl)phenthiazine-2-sulfonic acid dimethylamide.
10-(3-Methanesulphonyloxypropyl)phenthiazine-2-sulfonic acid dimethylamide and 4-hydroxyethyl piperidine in toluene were heated under reflux with stirring. The reaction mixture was allowed to cool and water was added. The resulting toluene solution layer was decanted and washed twice with water. The toluene solution was then stirred with 5% hydrochloric acid. The hydrochloride of the desired phenthiazine base precipitated in gummy condition in the aqueous layer. This was decanted and treated with sodium hydroxide. It was then extracted three times with ethyl acetate. The extracts were dried over sodium sulfate, filtered and concentrated in vacuum. A resinous product was obtained. This product was dissolved in a mixture of benzene and cyclohexane and chromatographed on a column containing alumina. The chromatographed product was eluted successively with mixtures of benzene and cyclohexane and then with benzene and finally with a mixture of benzene and ethyl acetate. The eluates were evaporated to yield a crude product. This product was recrystallised from aqueous ethanol and yielded 10[3-[4-(2-hydroxyethyl)piperidyl]propyl]phenthiazine-2-sulfonic acid dimethylamide.
Therapeutic Function
Neuroleptic
Pharmacokinetics
Pipotiazine has actions similar to those of other phenothiazines. Among the different phenothiazine derivatives, it appears to be less sedating and to have a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. However, it produces a high incidence of extra pyramidal reactions. It reduces activity of dopamine receptors in the limbic system. Its 5-HT antagonism helps normalize dopamine activity in the cortical regions.
Metabolism
Not Available
Properties of Pipotiazine
| Boiling point: | 650.4±65.0 °C(Predicted) |
| Density | 1.236±0.06 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), Methanol (Sparingly) |
| pka | 15.10±0.10(Predicted) |
| form | Solid |
| color | Pale Yellow to Light Yellow |
Safety information for Pipotiazine
Computed Descriptors for Pipotiazine
Pipotiazine manufacturer
Ralington Pharma
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![2-[1-[3-[2-[(dimethylamino)sulphonyl]-10H-phenothiazin-10-yl]propyl]piperidin-4-yl]ethyl palmitate](https://img.chemicalbook.in/CAS/GIF/37517-26-3.gif)
You may like
-
 39860-99-6 Pipothiazine 98%View Details
39860-99-6 Pipothiazine 98%View Details
39860-99-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1