Piperonyl aldehyde
Synonym(s):1,3-Benzodioxole-5-carboxaldehyde;3,4-(Methylenedioxy)benzaldehyde;Heliotropin
- CAS NO.:120-57-0
- Empirical Formula: C8H6O3
- Molecular Weight: 150.13
- MDL number: MFCD00005828
- EINECS: 204-409-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:38
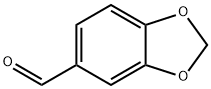
What is Piperonyl aldehyde?
Chemical properties
white crystalline solid
Chemical properties
Heliotropin occurs in a number of essential oils, but only in low concentrations. It forms white crystals (mp 37°C) with a sweet, floral, slightly spicy, heliotrope-like odor.
Chemical properties
Piperonal has a sweet, flowery odor reminiscent of heliotrope and a bittersweet taste.
Occurrence
Reported found in the essential oils of Robinia pseudo-acacia and Eryngium poterium; in the oils of Spirea ulmaria and of leaves of Doryphora sassafras; also reported found in Tahitian and Bourbon vanilla, camphor wood oil, violet flowers concrete and absolute, burley tobacco, rabbiteye blueberry, melon, pepper, cooked chicken, sherry and dill.
The Uses of Piperonyl aldehyde
Piperonal is an impurity of Tadalafil (T004500). Tadalafil impurity A.
The Uses of Piperonyl aldehyde
In perfumery, in cherry and vanilla flavors, in organic syntheses.
The Uses of Piperonyl aldehyde
Piperonal is used as fragrance and flavoring agent.
Definition
ChEBI: An arenecarbaldehyde that is 1,3-benzodioxole substituted by a formyl substituent at position 5. It has been isolated from Piper nigrum.
Preparation
Heliotropin is produced by two main routes:
1) From isosafrole: For many years, oxidative cleavage of isosafrole was the only
route applicable on an industrial scale. Isosafrole [120-58-1] is obtained by
isomerization from safrole [94-59-7], which can be isolated from (Chinese)
sassafras oil . Examples of oxidants that give good yields of
heliotropin are chromium(VI) salts, oxygen, and ozone.
This method is still used currently, but the destructive exploitation of sassafras
trees in Southeast Asia has led to a strong decline in the availability of
sassafras oil and thus of safrole/isosafrole.
2) From catechol: Several routes have recently been developed for the synthesis
of heliotropin from catechol. In one such route, catechol is converted into
3,4-dihydroxymandelic acid with glyoxylic acid in an alkaline medium in the
presence of aluminum oxide. 3,4-Dihydroxymandelic acid is oxidized to the
corresponding keto acid (e.g., with copper-(II) oxide), which is decarboxylated
to 3,4-dihydroxybenzaldehyde. The latter product is converted
into heliotropin, for example, by reactionwith methylene chloride in the presence
of quaternary ammonium salts.
In another route, catechol is first reacted with methylene chloride and converted
into 1,2-methylenedioxybenzene . Reaction with glyoxylic acid in
strongly acidic media yields 3,4-methylenedioxymandelic acid . Subsequent
oxidation and decarboxylation with nitric acid afford heliotropin.
Alternative routes that start from 1,2-methylenedioxybenzene and use
piperonyl chloride as intermediate have been described .
Aroma threshold values
Detection: 62 ppb to 1 ppm. Aroma characteristics at 1.0%: sweet, anise-like, almond vanilla, floral, black cherry pit, berry raspberry, powdery coumarin-like with a hint of hay.
Taste threshold values
Taste characteristics at 10 to 50 ppm: ripe black cherry fleshy, ripe berry, sweet, macaroon, Jordan almond, creamy vanilla, spicy cream soda, courmarin, slight floral with hay nuances.
Synthesis Reference(s)
Canadian Journal of Chemistry, 64, p. 225, 1986 DOI: 10.1139/v86-039
The Journal of Organic Chemistry, 48, p. 4053, 1983 DOI: 10.1021/jo00170a036
Tetrahedron Letters, 33, p. 5909, 1992 DOI: 10.1016/S0040-4039(00)61086-9
General Description
Colorless lustrous crystals.
Air & Water Reactions
Slightly water soluble .
Reactivity Profile
Piperonyl aldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Piperonyl aldehyde is sensitive to light. Piperonyl aldehyde may react with oxidizing materials.
Fire Hazard
Flash point data for Piperonyl aldehyde are not available. Piperonyl aldehyde is probably combustible.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Can cause central nervous system depression. A human skin irritant. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. See also ALDEHYDES.
Synthesis
By the oxidation of isosafrole with potassium dichromate and sulfuric acid and subsequent steam distillation of piperonal
Metabolism
In the animal body heliotropin undergoes the expected metabolic reaction involving oxidation to the corresponding acid (Williams, 1959).
Purification Methods
Crystallise piperonal from aqueous 70% EtOH or EtOH/water. [Beilstein 19/4 V 225.]
Properties of Piperonyl aldehyde
| Melting point: | 35-39 °C(lit.) |
| Boiling point: | 264 °C(lit.) |
| Density | 1.2645 (rough estimate) |
| vapor pressure | 1 mm Hg ( 87 °C) |
| refractive index | 1.4500 (estimate) |
| FEMA | 2911 | PIPERONAL |
| Flash point: | >230 °F |
| storage temp. | Dark Room |
| solubility | methanol: 0.1 g/mL, clear |
| form | A crystalline solid |
| Odor | at 100.00 %. heliotrope flower sweet powdery coconut vanilla |
| Water Solubility | Slightly soluble |
| Sensitive | Air & Light Sensitive |
| JECFA Number | 896 |
| Merck | 13,7556 |
| BRN | 131691 |
| Stability: | Stable, but air and light sensitive. Combustible. Incompatible with strong oxidizing agents, bases. |
| CAS DataBase Reference | 120-57-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Piperonal(120-57-0) |
| EPA Substance Registry System | Piperonal (120-57-0) |
Safety information for Piperonyl aldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Piperonyl aldehyde
Piperonyl aldehyde manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
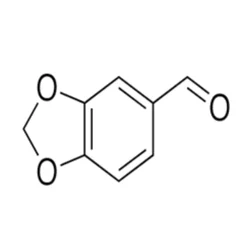 Piperonal (120-57-0) (C8H6O3), 95-99.99 %, PowderView Details
Piperonal (120-57-0) (C8H6O3), 95-99.99 %, PowderView Details
120-57-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
