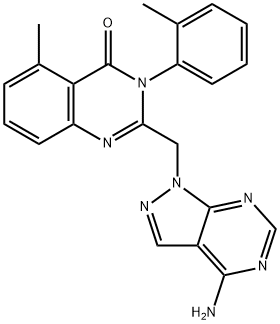
PIK-294
Synonym(s):2-((4-Amino-3-(3-hydroxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)methyl)-5-methyl-3-o-tolylquinazolin-4(3H)-one;2-[[4-Amino-3-(3-hydroxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]methyl]-5-methyl-3-(2-methylphenyl)-4(3H)-quinazolinone;PIK294
- CAS NO.:900185-02-6
- Empirical Formula: C28H23N7O2
- Molecular Weight: 489.53
- MDL number: MFCD17019338
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
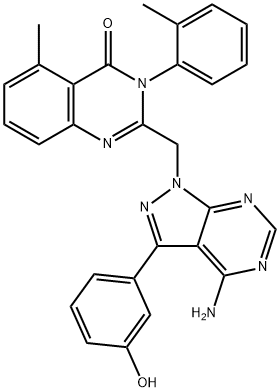
What is PIK-294?
The Uses of PIK-294
PIK-294 is a derivative of PIK-293 (P437700) and a highly selective inhibitor of p110δ which is an isoform of phosphoinositide 3-kinase (PI3K).
What are the applications of Application
PIK-294 is a selective PI3K inhibitor
Biological Activity
pik-294 is a highly selective p110δ inhibitor, 1000-, 49- and 16-fold less potent to pi3kα/β/γ, respectively.phosphoinositide 3-kinases (pi3-ks) are a key emerging class of drug targets, but the unique roles of pi3-k isoforms remain rarely defined. their target selectivity was biochemically enumerated that revealed cryptic homologies across targets and chemotypes by synthesizing chemically diverse panel of pi3-k inhibitors. crystal structures of three inhibitors to p110g identify a conformationally mobile region that is uniquely exploited by bound selective compounds. this chemical array was then used to define the pi3-k isoforms required for insulin signaling.
Biochem/physiol Actions
PIK-294 is a highly selective inihibitor of the phosphoinositide 3-kinase (PI3K) p110δ with an IC50 value of 10 nM compared to IC50 values of 10 μM, 490 nM and 160 nM for PI3Kα/β/γ, respectively. PIK-294 has been used to help distinguish the unique roles of the various PI3-K isoforms. For example, in a study of CXCL8-induced neutrophil migration, PIK-294 inhibited both chemokinetic and chemotactic CXCL8-induced migration whereas PI3Kγ inhibitor AS-605240 markedly reduced CXCL8 induced chemokinetic migration but had no effect on CXCL8 induced chemotactic migration.
in vitro
pik-294 displays distinct patterns of isoform selectivity to inhibit different subsets of class i pi3k isoforms (p110β, p110δ, and p110γ) and shows low sensitivity to p110α with ic50 of 10 μm). the m-phenol moiety of pik-294 can penetrate the deep-affinity pocket of the atp binding site, and thus promotes in vitro inhibitory activity. pik-294 showed one of the most potent p110d-selective inhibitors reported at present.
in vivo
pik-294 bound p110a inhibits the acute effects of insulin treatment in vivo, whereas a p110b inhibitor has no effect.
References
[1] knight za, gonzalez b, feldman me, zunder er, goldenberg dd, williams o, loewith r, stokoe d, balla a, toth b, balla t, weiss wa, williams rl, shokat km, et al. . a pharmacological map of the pi3-k family defines a role for p110alpha in insulin signaling. cell. 2006 may 19;125(4):733-47. epub 2006 apr 27.
[2] bobrovnikova-marjon e1, pytel d, riese mj, vaites lp, singh n, koretzky ga, witze es, diehl ja. perk utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate akt activation, and promote adipocyte differentiation. mol cell biol. 2012 jun;32 (12):2268-78.
Properties of PIK-294
| Boiling point: | 790.4±70.0 °C(Predicted) |
| Density | 1.43 |
| storage temp. | -20°C |
| solubility | ≥24.5 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| pka | 8.95±0.10(Predicted) |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 900185-02-6 |
Safety information for PIK-294
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for PIK-294
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N-(2,3-Dihydro-7,8-dimethoxyimidazo[1,2-c]quinazolin-5-yl)-3-pyridinecarboxamide](https://img.chemicalbook.in/CAS/GIF/677338-12-4.gif)
You may like
-
 PIK-294 >95% CAS 900185-02-6View Details
PIK-294 >95% CAS 900185-02-6View Details
900185-02-6 -
 PIK-294 CAS 900185-02-6View Details
PIK-294 CAS 900185-02-6View Details
900185-02-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8