phenyltoloxamine
- CAS NO.:92-12-6
- Empirical Formula: C17H21NO
- Molecular Weight: 255.35
- EINECS: 202-127-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
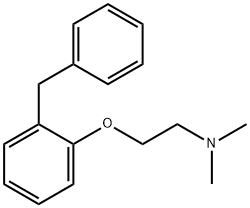
What is phenyltoloxamine?
Absorption
Readily accessible data regarding the absorption of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated .
Toxicity
Toxicity after overdose may involve CNS effects like extreme drowsiness, lethargy, confusion, delirium, and coma in adults; paradoxical excitation, irritability, hyperactivity, insomnia, hallucinations, seizures, and respiratory depression or arrest in infants and young children, CNS adverse effects predominate o er cardiac adverse effects; death may occur within hours after ingestion of drug in untreated patients; rhabdomyolysis has also been reported .
Originator
Bristalin, Bristol ,US,1952
Background
Phenyltoloxamine is an antihistamine drug with sedative and analgesic effects. It is a H1 receptor blocker and a member of the ethanolamine class of antihistaminergic drugs. It is available in combination products that also contain other analgesics and antitussives such as acetaminophen. Phenyltoloxamine citrate is the more common salt form that acts as an active ingredient in pharmaceutical products and promotes hay fever relief via reversing the effects of histamine. Phenyltoloxamine acts as an adjuvant analgesic, which augments the analgesic effect of acetaminophen. It also potentiates the effects of other drugs, such as codeine and codeine derivatives.
Although phenyltoloxamine's ability to potentiate the effects of analgesics may be explained in part by its chemical nature as a first-generation H1 antihistamine that is capable of crossing the blood-brain barrier and causing tranquilizing effects at CNS histamine receptors, many of the drug's specific pharmacokinetics are not readily available - perhaps also because many early (phenyltoloxamine was involved in studies as early as the 1950s) first-generation antihistamines were not optimally investigated . Nevertheless, phenyltoloxamine is used to a fairly limited extent in contemporary medicine, with only very few products involving it as an active ingredient.
Indications
The primary therapeutic use for which phenyltoloxamine is currently indicated is as an adjuvant therapy in various combination products containing an analgesic(s) (either narcotic or non-narcotic), where it is expected to potentiate the pain relieving, anti-tussive, etc. effect(s) of the analgesic component of the product.
In that regard, some of these aforementioned combination products are typically indicated for the temporary relief of minor aches and pains like headache, muscular aches, backaches, minor arthritis pain, common cold, toothaches, menstrual cramps, etc ; or perhaps for the treatment of exhausting or non-productive cough, associated with cold or with upper respiratory allergic condition that does not respond to non-narcotic antitussives .
Definition
ChEBI: Phenyltoloxamine is a diarylmethane.
Manufacturing Process
Sodium methylate is made by dropping 11.7 g of sodium strips into 199 ml of absolute methanol in a 1-liter three-necked flask. 93.9 g of o-benzylphenol are dissolved in 200 ml of dry toluene and added to the sodium methylate solution. The solution is distilled until the boiling point of toluene is reached. At the end of the distillation, enough toluene is added to restore the original volume of solvent.
109.5 g of dimethylaminoethyl chloride hydrochloride and 200 ml of toluene are placed in a 1-liter Erlenmeyer flask, cooled in an ice bath, and decomposed with 167.5 g of 20% sodium hydroxide solution. The toluene and water layers are separated, and the water layer is extracted again with 50 ml of toluene. The toluene layers are combined, washed with saturated salt solution, and dried over anhydrous potassium carbonate.
The dried dimethylaminoethyl chloride solution is poured into the toluene solution of the sodium salt of o-benzylphenol, heated to reflux, and refluxed 16 hours. After refluxing, enough water is added to the mixture to dissolve the precipitated solid. The layers are separated, and the toluene layer is further washed with water until the water extract is just slightly alkaline. The toluene solution is then made acid with 6N hydrochloric acid and extracted with water until no cloudiness is produced when the extract is made alkaline. The acidic aqueous extract is washed with ether, then made alkaline with 20% sodium hydroxide solution, and extracted into ether. The ether solution is washed several times with water, then with saturated salt solution, and is dried over anhydrous potassium carbonate. The dried solution is filtered and distilled. The product distills at 143.5°C/1 mm; 69.7 g of pale yellow oil are recovered.
57.1 g of the free base are dissolved in ether and precipitated with dry HCl. 66.0 g of crude hydrochloride are recovered. The hydrochloride is dissolved in 130 ml of reagent acetone by boiling, filtered hot, and allowed to cool. The crystalline material obtained on cooling is filtered, washed with a little acetone, washed with ether, and dried in vacuo. 44.8 g, MP 119.5°C to 121°C, are recovered from the first crop of crystals. Ethyl acetate may also be used as the solvent for recrystallization.
Therapeutic Function
Antihistaminic
Pharmacokinetics
As a member of the first generation H1 antihistamines, it is known that phenyltoloxamine - like virtually all first generation H1 antihistamines - has a propensity for crossing the blood-brain barrier and acting on H1 histamine receptors there to interfere with neurotransmission . The most common results of this kind of first generation H1 antihistamine CNS neurotransmission interference are adverse effects like drowsiness, sedation, somnolence, and fatigue . Given these effects, under specific circumstances like a patient experiencing a pain or a cough that may be preoccupying all of their waking energy and attention, it is perhaps possible that the sedative and tranquilizing characteristics of phenyltoloxamine may be the factors that contribute to its apparent adjunctive analgesic and antitussive actions .
Metabolism
Readily accessible data regarding the metabolism of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated .
Properties of phenyltoloxamine
| Melting point: | <25 °C |
| Boiling point: | bp 141-144° (at <0.1 mm Hg) |
| Density | 1.022±0.06 g/cm3(Predicted) |
| pka | pKa 9.1 (Uncertain) |
| EPA Substance Registry System | Phenyltoloxamine (92-12-6) |
Safety information for phenyltoloxamine
Computed Descriptors for phenyltoloxamine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
