Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
Synonym(s):BAPOs;Bisacylphosphine oxides
- CAS NO.:162881-26-7
- Empirical Formula: C26H27O3P
- Molecular Weight: 418.47
- MDL number: MFCD01863675
- EINECS: 423-340-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:21
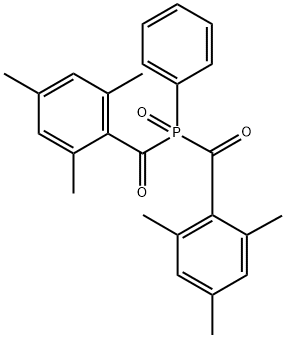
What is Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide?
Description
Phenylbis(2,4,6-trimethylbenzoyl) phosphine oxide can be used as a photoinitiator for: Radical polymerization of dental resins. It enhances the polymerization rate and conversion compared to other initiators. It is a polymer-based ceramic material modified with divinylbenzene for the preparation of high-temperature sensor applications.
The Uses of Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide is a useful research chemical compound.
The Uses of Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
Versatile UV photoinitiator for radical polymerization of unsaturated resins, especially pigmented formulations.
Preparation
Under nitrogen protection and room temperature, 1.4 g of zinc powder was added to the 100 mL reaction flask with stirring.15 ml of ethyl acetate, 4.1 g of 2,4,6-trimethyl benzoyl chloride, and a solution of 2.5 g of phenylphosphorus dichloride in 5 ml of ethyl acetate was added dropwise to the mixture. After that, the temperature was continued for 2 hours. After the completion of the reaction, 20 mL of water and 1.5 mL of 30% hydrogen peroxide were respectively added to the system, and the reaction was continued for half an hour. Saturated sodium bicarbonate solution was added to neutralize weakly alkaline. Finally, Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide was obtained after purification.
Definition
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide (PPO) has a local absorption maximum in the visible spectrum at 405 nm and could provide sufficient initiating radicals to crosslink polyamic diacrylate ester in NMP[1]. Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide (GR-XBPO) could be used as the reductant, and Monoethanolamine as the oxygen inhibition agent. After UV irradiation, oxygen-containing groups (OCGs) on the graphene oxide (GO) plane and edges are largely removed due to the reduction of GO by free radicals generated by photoinitiator decomposition[2].
References
[1] Maruti Hegde. “3D Printing All-Aromatic Polyimides using Mask-Projection Stereolithography: Processing the Nonprocessable.” Advanced Materials 29 31 (2017).
[2] Bing Xue, Yuchun Yang, Yingquan Zou. “A UV-light induced photochemical method for graphene oxide reduction.” Journal of Materials Science 52 21 (2017): 12742–12750.
What are the applications of Application
Photoinitiator 819 (HRcure-819) can also be used in colored UV-curable plastic coatings. UV coatings have been widely used on plastic shells of various electronic and household appliances due to their excellent performance and efficient production. However, the deep curing of UV coatings after coloring is not good, resulting in poor film adhesion and poor dispersion and arrangement of pigments by UV resins, which seriously affect the appearance of the coatings. Therefore, the traditional construction process is to paint solvent-based colored primer first; after baking, apply UV varnish to improve the physical properties of the film surface.
Properties of Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
| Melting point: | 131-135 °C(lit.) |
| Boiling point: | 590.0±60.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | acetone, acetonitrile, toluene, and hexanedioldiacrylate: soluble |
| form | powder |
| color | Pale Yellow to Yellow |
| Water Solubility | 100μg/L at 20-21℃ |
| λmax | 366nm(MeOH)(lit.) |
| InChI | InChI=1S/C26H27O3P/c1-16-12-18(3)23(19(4)13-16)25(27)30(29,22-10-8-7-9-11-22)26(28)24-20(5)14-17(2)15-21(24)6/h7-15H,1-6H3 |
| CAS DataBase Reference | 162881-26-7 |
| EPA Substance Registry System | Phosphine oxide, phenylbis(2,4,6-trimethylbenzoyl)- (162881-26-7) |
Safety information for Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
| InChIKey | GUCYFKSBFREPBC-UHFFFAOYSA-N |
| SMILES | P(C1C=CC=CC=1)(=O)(C(C1C(=CC(C)=CC=1C)C)=O)C(C1C(=CC(C)=CC=1C)C)=O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Phenylbis(2,4,6-trimethylbenzoyl)phosphine Oxide CAS 162881-26-7View Details
Phenylbis(2,4,6-trimethylbenzoyl)phosphine Oxide CAS 162881-26-7View Details
162881-26-7 -
 Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide, 99% CAS 162881-26-7View Details
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide, 99% CAS 162881-26-7View Details
162881-26-7 -
 Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide 98% (HPLC) CAS 162881-26-7View Details
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide 98% (HPLC) CAS 162881-26-7View Details
162881-26-7 -
 Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide CAS 162881-26-7View Details
Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide CAS 162881-26-7View Details
162881-26-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
