PHENTERMINE
- CAS NO.:122-09-8
- Empirical Formula: C10H15N
- Molecular Weight: 149.24
- MDL number: MFCD00059195
- EINECS: 204-522-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:51
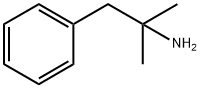
What is PHENTERMINE?
Absorption
Phentermine shows a dose-dependent pharmacokinetic profile. After oral administration of a dose of 15 mg, the maximal concentration was achieved after 6 hours and its bioavailability was not affected by the consumption of high-fat meals. The reported plasma concentration at steady-state is of around 200 ng/ml as observed in clinical trials.
Toxicity
The reported LD50 after oral administration of phentermine in rats is reported to be of 151 mg/kg. Reports of acute overdose include restlessness, tremors, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations and panic state followed by fatigue, and depression. In the cardiovascular system, there are reports of tachycardia, arrhythmia, hypertension, hypotension, circulatory collapse. In the GI tract, there are symptoms of nausea, vomiting, diarrhea and abdominal cramps. The management of acute overdosage includes symptomatic treatment as well as lavage and sedation with barbiturates.
On the other hand, chronic overdosage is marked by dermatoses, insomnia, irritability, hyperactivity and personality changes. In severe cases, it can derive into a schizophrenia-like psychosis.
Studies regarding the carcinogenic potential have not been performed. On the case of mutagenic assays, phentermine was shown to not be mutagenic nor clastogenic.
Description
Phentermine, in combination with fenfluramine, formed the notorious appetite-suppressing drug Fen-Phen, which was withdrawn from the market in 1997 because it produced heart valve disease in two dozen patients. Before the development of Fen-Phen, phentermine was used as a cation-exchange resin complex in other diet drugs. It synthesis was first reported in 1946 by R. S. Shelton and M. G. Van Campen.
Originator
Wilpo ,Dorsey, US,1961
The Uses of PHENTERMINE
Appetite suppressant (systemic).
Indications
Phentermine is indicated, alone or in combination with topiramate, as a short-term adjunct, not pass a few weeks, in a regimen of weight reduction based on exercise, behavioral modifications and caloric restriction in the management of exogenous obesity for patients with an initial body mass index (BMI) greater than 30 kg/m2 or greater than 27 kg/m2 in presence of other risk factors such as controller hypertension, diabetes or hyperlipidemia.
Exogenous obesity is considered when the overweight is caused by consuming more food than the person activity level warrants. This condition commonly causes an increase in fat storage. It is an epidemic condition in the United States where over two-thirds of adults are overweight or obese and one in three Americans is obese. In the world, the incidence of obesity has nearly doubled.
Background
Phentermine is a sympathomimetic amine anorectic agent and it was introduced in 1959 as part of an anti-obesity combination drug. It is chemically related to amphetamine and it is commonly referred to as an atypical amphetamine. Phentermine has not been reported an addictive potential which allows this agent to be classified under the Schedule IV drugs (low abuse potential).
Phentermine was FDA approved for short-term weight management in 1959 and it became widely used in 1960. This initial product, formed by the combination of phentermine with fenfluramine and dexfenfluramine was discontinued after finding several reports of abnormal valves in nearly 30% of the consumers. Later on, phentermine was approved alone and in combination with topiramate in 2012 as a new alternative that required lower doses of phentermine to obtain the desired effect.
Definition
ChEBI: Phentermine is a primary amine. It has a role as a dopaminergic agent, an adrenergic agent, a sympathomimetic agent, an appetite depressant, a central nervous system stimulant and a central nervous system drug. It is a conjugate base of a phentermine(1+).
Manufacturing Process
Preparation of isobutyrophenone: In a 12 liter, 3-necked flask, 1,280 grams of aluminum chloride was covered with 2,000 cc of dry thiophene-free benzene and a solution of 919 grams of isobutyryl chloride, (BP 92°-94°C) in 1 liter of benzene was added slowly with stirring. After heating for 3 hours at reflux, the solution was cooled and poured over a mixture of 1 liter of concentrated hydrochloric acid and 5 kg of ice. The benzene layer was separated, the aqueous layer extracted with benzene, and the combined benzene solutions were washed, dried and concentrated in vacuo. The residue was distilled rapidly to give 1,051 grams of isobutyrophenone, boiling at 81°-89°C at 1 mm, yield 83.4%.
Preparation of 1,3-Diphenyl-2,2-Dimethylpropanone-1: Sodamide was prepared from 12.5 grams of sodium added in small portions to 600 cc of liquid ammonia with 1 gram of hydrous ferric chloride as catalyst. The ammonia was replaced by 200 cc of dry toluene and without delay a solution of 74 grams of isobutyrophenone and 76.5 grams of benzyl bromide in 200 cc of benzene was slowly added with stirring. The reaction mixture was heated on a boiling water bath for 48 hours. Water was then added, the organic layer separated and the product isolated by distillation. The 1,3-diphenyl-2,2
Phentermine dimethylpropanone-1 boiled from 142°-143°C at a pressure of 3 mm, nD201.5652.
Preparation of α,α-Dimethyl-β-Phenylpropionamide: Sodamide was prepared from 7.6 grams of sodium in 350 cc of liquid ammonia with 0.9 gram of hydrous ferric chloride. The ammonia was replaced by 250 cc of toluene, the mixture was heated to 60°C and 71.4 grams of 1,3-diphenyl-2,2-dimethyl propanone-1 dissolved in 150 cc of toluene was added. The mixture was stirred and heated on a steam bath for 5 hours. A clear red color appeared in 15 minutes and disappeared after about an hour. After cooling, water was added, the organic layer was washed, dried, and concentrated to give 36.5 grams of α,α-dimethyl-β-phenyl propionamide which crystallized slowly after the addition of an equal volume of petroleum ether. The product melted at 62°C after crystallization from benzene-petroleum ether.
Preparation of Di-(β-Phenyl-α,α-Dimethylethyl)Urea: 3.5 grams of α,αdimethyl-β-phenylpropionamide in 420 cc of water was added to a solution of 87.5 grams of potassium hydroxide and 35 grams of bromine in 350 cc of water. After 2 hours at 60°C, the product was obtained on crystallization from ethanol, melting at 184°C.
Preparation of ω-Phenyl-tert-Butylamine: 24 grams of the urea derivative obtained as indicated above, were well mixed with 96 grams of calcium hydroxide in a flask immersed in an air bath and provided with a dropping funnel the stem of which reached the bottom of the flask. The mixture was heated to 240°-260°C (inside temperature) for 7 hours during which time 86 cc of water was slowly added. The vapors were collected in a receiver cooled with ice. After extraction with ether and distillation, the product was obtained as a colorless liquid boiling from 80°-84°C at 9 mm according to US Patent 2,590,079.
The ether solution may be dried and saturated with hydrogen chloride and the precipitated hydrochloride recrystallized from a mixture of 50 parts alcohol and 100 parts of acetone.
The pure hydrochloride is thus obtained as a white crystalline substance having a MP of 195°-196°C, according to US Patent 2,408,345.
brand name
Ionamin (Fisons);Adipex nouveau;Adipex-p;Aneroxina;Bellapront;Dapex;Ex-adipos;Fastin;Inonamin;Ionakraft;Ionamine;Levum;Minobese forte;Mirapront;Netto-longcaps;Obestin 30;Oby-trim;Ona-mast;Panbesy;Panshape;Parmine;Phentermyl;Pronidin;Raucherstop 5 ht;Reducyl;Regulin;Span r/d;Teramine.
Therapeutic Function
Antiobesity
World Health Organization (WHO)
Phentermine, a sympathomimetic amine, was introduced in 1959 for use as an anorexic agent. It retains a place in the treatment of obesity. However,since it has been subject to abuse and because dependence can occur, phentermine is controlled under Schedule IV of the 1971 Convention on Psychotropic Substances. (Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV), , , 1971)
Synthesis Reference(s)
Journal of the American Chemical Society, 70, p. 4048, 1948 DOI: 10.1021/ja01192a023
General Description
Oily liquid. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
PHENTERMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides.
Pharmacokinetics
It is reported that the main mechanism of action of phentermine is the generation of appetite suppression, maybe due to the increase in leptin, but it is considered that other mechanisms should be involved. Some reports have indicated that the weight loss effect is mainly due to the increase in resting energy expenditure.
In clinical studies where phentermine was used as a monotherapy and as combination therapy, this drug has shown an average weight loss of 3.6 kg when compared with the placebo in 2-24 weeks. Patients treated with phentermine also showed increased maintenance of the weight after treatment discontinuation. As well, even though it is a derivative of the amphetamines, it has not been registered to produce any of the effects of amphetamine such as central nervous system stimulation, elevation of blood pressure, tachyphylaxis or QTc prolongation.
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: sympathomimetic. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx
Synthesis
Phentermine is prepared by converting the
condensation product of benzaldehyde and
nitropropane to the corresponding aminoalcohol
followed by transformation to the chloroamine
and then catalytic hydrogenation
.
Metabolism
Phentermine undergoes minimal p-hydroxylation, N-oxidation and N-hydroxylation followed by conjugation. The total proportion of the drug that goes under metabolism only represents about 6% of the administered dose where about 5% is represented by the N-oxidized and N-Hydroxylated metabolites.
Properties of PHENTERMINE
| Melting point: | 143°C (estimate) |
| Boiling point: | bp750 205°; bp21 100° |
| Density | 0,93 g/cm3 |
| vapor pressure | 32.4Pa at 25℃ |
| refractive index | 1.5090 (estimate) |
| Flash point: | 100 °C |
| storage temp. | Store at 0-5°C |
| solubility | Methanol: 50 mg/ml |
| form | Oil |
| pka | pKa 10.11 (Uncertain) |
| color | Colourless to Pale Yellow |
| Water Solubility | 18.6g/L at 25℃ |
| EPA Substance Registry System | .alpha.,.alpha.-Dimethylphenethylamine (122-09-8) |
Safety information for PHENTERMINE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for PHENTERMINE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4