petalite
- CAS NO.:1302-66-5
- Empirical Formula: AlH6LiO5Si2
- Molecular Weight: 176.13
- MDL number: MFCD01773667
- EINECS: 215-104-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-17 21:30:02
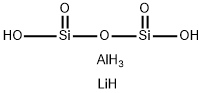
What is petalite?
Description
Petalite has the chemical formula LiAlSi4Oi0 . Petalite theoretically contains 2.27% lithium (4.88% lithia) but in nature contains about 1.8% lithium (3.5-4.5% lithia). The mineral is found in Sweden, South-west Africa and southern Rhodesia. Petalite is presently used as a source of lithium in glasses and glazes and as a low-expansion filler in refractories.
Chemical properties
Petalite, LiAlSi4O10, is a monoclinic mineral with a framework silicate structure. It is grayish white in color and occasionally has a pinkish hue. The mineral has two cleavage directions, which form an angle of 38.5°. The basal cleavage is perfect.
Physical properties
Petalite is a lithium sodium aluminum silicate with a hardness of 6 to 6.5. It belongs to the monoclinic crystal system. This mineral crystallizes relatively rarely and is commonly found in the form of large, cleavable masses. Petalite can exhibit various colors including white, colorless, gray, pinkish, or yellow. Colorless specimens can be cut into exceptionally brilliant gemstones and some can be shaped into cats-eyes. Petalite typically forms within granite pegmatites and is often found in association with other lithium-containing minerals such as Amblygonite, Kunzite, Spodumene, and Lepidolite. It is primarily sourced from Australia, Brazil, Sweden, Namibia, and Afghanistan.
Occurrence
The theoretical lithium content of petalite is 2.27%. In commercial deposits, the lithium content ranges from 1.6% to 2.1%. Petalite is found in significant quantities in conjunction with lepidolite in Zimbabwe (Bikita), Namibia (Karibib), Brazil (Aracuai), Australia (Londonderry), the former U.S.S.R. (eastern Transbaikalia), Sweden (Utoe), and Canada (Bernic Lake).
In certain pegmatites, there is evidence that petalite undergoes alteration to a mixture of spodumene and quartz. Cerny and Turnock (1971) described pseudomorphs of spodumene and quartz after petalite in the Bernic Lake pegmatites of Manitoba. This phenomenon, commonly referred to as SQI, occurs according to the following reaction: petalite (spodumene + 2 quartz).
Properties of petalite
| CAS DataBase Reference | 1302-66-5 |
Safety information for petalite
Computed Descriptors for petalite
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran







You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1