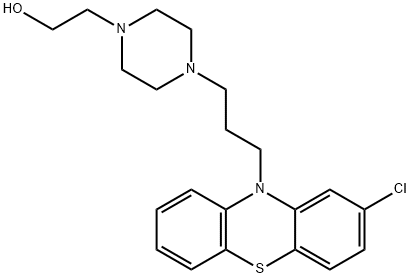
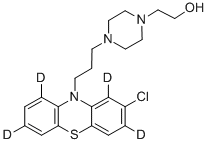
PERPHENAZINE
Synonym(s):4-[3-(2-Chloro-10H-phenothiazin-10-yl)propyl]-1-piperazineethanol;Perphenazine
- CAS NO.:58-39-9
- Empirical Formula: C21H26ClN3OS
- Molecular Weight: 403.97
- MDL number: MFCD00056798
- EINECS: 200-381-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is PERPHENAZINE?
Absorption
Absolute bioavailability is 40% following oral administration.
Toxicity
Symptoms of overdose include stupor or coma, and children may have convulsive seizures. Signs of arousal may not occur for 48 hours. Oral LD50=318 mg/kg (rat); IPR LD50=64 mg/kg (mouse)
Description
Perphenazine (58-39-9) is a clinically useful typical antipsychotic drug. The therapeutic mode of action is not well understood but it binds to a wide variety of receptors including serotonin, histamine, dopamine, and α-adrenergic.1 Perphenazine is an inhibitor of acid sphingomyelinase(ASM)2 and positively affected xenografted tumor growth via perturbation of intracellular cholesterol transport through ASM3. It has also been shown to be an inhibitor of glutamate dehydrogenase.4
Chemical properties
Off-White to Pale Yellow Solid
Originator
Trilafon, Schering ,US ,1957
The Uses of PERPHENAZINE
immune suppressant, antifungal
The Uses of PERPHENAZINE
D2 dopamine receptor antagonist; α-adrenergic receptor antagonist and σ-receptor agonist; phenothiazine antipsychotic. Inhibits glutamate dehydrogenase in vitro. Antipsychotic.
Indications
For use in the management of the manifestations of psychotic disorders and for the control of severe nausea and vomiting in adults.
Background
An antipsychotic phenothiazine derivative with actions and uses similar to those of chlorpromazine.
What are the applications of Application
Perphenazine is a D2DR inhibitor
Definition
ChEBI: A phenothiazine derivative in which the phenothiazine tricycle carries a chloro substituent at the 2-position and a 3-[4-(2-hydroxyethyl)piperazin-1-yl]propyl group at N-10.
Manufacturing Process
A mixture of 155 parts of 2-chloro-10-(γ-chloropropyl)phenothiazine, 76 partsof sodium iodide, 216 parts of piperazine and 2,000 parts of butanone is refluxed for 8 hours, concentrated and extracted with dilute hydrochloric acid. The extract is rendered alkaline by addition of dilute potassium carbonate and benzene or chloroform extracted. This extract is washed with water, dried over anhydrous potassium carbonate, filtered and evaporated. Vacuum distillation at 0.1 mm pressure yields 2-chloro-10-[γ-(N-piperazino)propyl]phenothiazine at about 214°-218°C.
A stirred mixture of 5 parts of 2-chloro-10-[γ-(Npiperazino)propyl]phenothiazine, 1.92 parts of 2-bromoethanol, 2.11 parts of potassium carbonate and 35 parts of toluene is refluxed for 5 hours. The mixture is treated with water and benzene and the organic layer is separated, washed with water, dried over anhydrous potassium carbonate, filtered and evaporated. The residue is distilled at about 240°-244°C and 0.15 mm pressure to yield 2-chloro-10-[γ-(N'-β-hydroxyethyl-N-piperazino)propyl]phenothiazine according to US Patent 2,838,507.
The 2-chloro-10-(γ-chloropropyl)phenothiazine starting material is produced from 2-chlorophenothiazine and 1-bromo-3-chloropropane.
brand name
Trilafon (Schering).
Therapeutic Function
Tranquilizer
General Description
Perphenazine, 4-[3-(2-chlorophenothiazine-10-yl)propyl]piperazineethanol; 2-chloro-10-[3-[4-(2-hydroxyethyl)piperazinyl]propyl]phenothiazine(Trilafon), is an effective antipsychotic and antiemetic.
Biochem/physiol Actions
D2 dopamine receptor antagonist; α-adrenergic receptor antagonist and σ-receptor agonist; phenothiazine antipsychotic. Inhibits glutamate dehydrogenase in vitro.
Pharmacokinetics
Perphenazine is a piperazinyl phenothiazine, acts on the central nervous system, and has a greater behavioral potency than other phenothiazine derivatives whose side chains do not contain a piperazine moiety. It is a member of a class of drugs called phenothiazines, which are dopamine D1/D2 receptor antagonists. Perphenazine is 10 to 15 times as potent as chlorpromazine; that means perphenazine is a highly potent antipsychotic. In equivalent doses it has approximately the same frequency and severity of early and late extrapypramidal side-effects compared to Haloperidol.
Safety Profile
Poison by ingestion, intravenous, subcutaneous, intraperitoneal, and intramuscular routes. Human systemic effects by intramuscular route: muscle spasms. Experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SOx, NOx, and cl-.
Metabolism
Hepatic.
References
1) Kroeze et al. (2003), H1-Histamine Receptor Affinity Predicts Short-Term Weight Gain for Typical and Atypical Antipsychotic Drugs; Neuropharmacology, 28 519 2) Kornhuber et al. (2011), Identification of Novel Functional Inhibitors of Acid Sphingomyelinase; PLoS One, 6 e23852 3) Kuzu et al. (2017), Modulating cancer cell survival by targeting intracellular cholesterol transport; Br. J. Cancer,?117 513 4) Couee and Tipton (1990), Inhibition of ox brain glutamate by perphenazine; Biochem. Pharmacol. 39 1167
Properties of PERPHENAZINE
| Melting point: | 95-98?C |
| Boiling point: | bp0.15 214-218°; bp1 278-281° |
| Density | 1.253 |
| refractive index | 1.6100 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in ethanol (96 per cent). It dissolves in dilute solutions of hydrochloric acid. |
| form | neat |
| pka | pKa 7.8(H2O,t =24±1) (Uncertain) |
| form | Solid |
| color | White |
| Water Solubility | 28.28mg/L(24 ºC) |
| Merck | 14,7183 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 58-39-9(CAS DataBase Reference) |
Safety information for PERPHENAZINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for PERPHENAZINE
PERPHENAZINE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Perphenazine 58-39-9 98%View Details
Perphenazine 58-39-9 98%View Details
58-39-9 -
 Perphenazine CAS 58-39-9View Details
Perphenazine CAS 58-39-9View Details
58-39-9 -
 Perphenazine 98% CAS 58-39-9View Details
Perphenazine 98% CAS 58-39-9View Details
58-39-9 -
 Perphenazine CAS 58-39-9View Details
Perphenazine CAS 58-39-9View Details
58-39-9 -
 Perphenazine CAS 58-39-9View Details
Perphenazine CAS 58-39-9View Details
58-39-9 -
 Perphenazine CAS 58-39-9View Details
Perphenazine CAS 58-39-9View Details
58-39-9 -
 Perphenazine CAS 58-39-9View Details
Perphenazine CAS 58-39-9View Details
58-39-9 -
 Perphenazine solution CAS 58-39-9View Details
Perphenazine solution CAS 58-39-9View Details
58-39-9