Perindopril erbumine
Synonym(s):(2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(Ethoxycarbonyl)butyl]amino]-1-oxopropyl]octahydro-1H-indole-2-carboxylic acid tert-butylamine salt;Perindopril tert-butylamine;Perindopril erbumine;S-9490
- CAS NO.:107133-36-8
- Empirical Formula: C23H43N3O5
- Molecular Weight: 441.61
- MDL number: MFCD02313824
- EINECS: 600-798-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
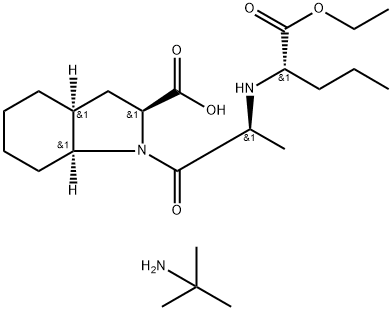
What is Perindopril erbumine?
Chemical properties
White Solid
Originator
Aceon ,Solvay Pharmaceuticals Inc. ,USA
The Uses of Perindopril erbumine
An angiotensin-converting enzyme (ACE) inhibitor. Antihypertensive.
The Uses of Perindopril erbumine
antibacterial
What are the applications of Application
Perindopril t-Butylamine Salt is an angiotensin-converting enzyme (ACE) inhibitor
Definition
ChEBI: Perindopril erbumine is an addition compound. It has a role as an antihypertensive agent and an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor. It contains a perindopril(1-).
Manufacturing Process
Heat 5 kg of 2-carboxyindole suspended in ethanol in the presence of sulfuric acid to boiling for 8 hours. Evaporate off take up the crystalline mass with hexane. After filtering off and drying, 5.3 kg of 2-ethoxycarbonylindole crystals are obtained. Melting point: 123°-125°C.
Suspend, in a reactor, 10 kg of 2-ethoxycarbonylindoline obtained previously in 110 liters of hydrochloric ethanol. Next, add 20 kg of granulated tin. Keep stirring for approximately 2 days at room temperature. Evaporate off the ethanol, take up the residue with water and add 110 liters of toluene. Stir for approximately 20 min. Alkalify with aqueous ammonia. Separate off the aqueous phase and extract once again with 150 liters of toluene. Combine the toluene phases and wash them with water. Separate off the toluene phases, filter. Remove the water by distilling the water-toluene azeotrope. Cool and pass through a stream of anhydrous HCl gas. Cool. Evaporate down and wash with pure toluene. Weight obtained of (R,S)-2-ethoxycarbonylindoline 10.11 kg. Yield: 84%.
2.15 kg of (R,S)-2-ethoxycarbonylindoline dissolved in ethanol are saponified with 12.5 liters of sodium hydroxide with stirring for 24 hours. After washing the alkaline solution, neutralize with concentrated hydrochloric acid. After filtering off, washing and drying, 1.57 kg of white crystals of the (R,S)-2carboxyindoline are obtained. Yield: 86%. Melting point: 188°-189°C.
6.05 kg of (R,S)-2-carboxyindoline are added to a solution of 4.49 kg of (+)α-methylbenzylamine in anhydrous ethanol. A white precipitated product is obtained which, after filtering off, is digested in refluxing isopropanol. After cooling, the solid is filtered off and washed with a little isopropanol. 1 kg of the obtained salt was dissolved in 5 liters of water and neutralizing with an aqueous hydrochloric acid solution. The precipitate is filtered off, washed with water and dried and (S)-2-carboxyindoline was prepared.
Place 25 kg of (S)-2-carboxyindoline, obtained previously, in 110 liters of methanol in a vessel. Keep stirred. Charge the rhodium (5% dry) catalyst into a mixer. Start up the stirring in a hydrogenator, charge the methanolic suspension of (S)-2-carboxyindoline by passing it through the mixer and rinse the assembly with water. Heat to 60°C and pressurize with hydrogen (30 bars). Filter off the catalyst on a single-plate filter. Collect the hydroalcoholic liquors in a reactor and evaporate the methanol off under vacuum. After concentrating, charge approximately 300 kg of dioxane. Heat to boiling and add water until a solution is obtained. Allow to cool. Filter off and dry. 22.3 kg of crystals of (2S,3aS,7aS)-2-carboxyoctahydroindole are obtained. Yield: 86.1%.
Place 35 kg of L-norvaline in approximately 300 kg of denatured ethanol in a reactor. Introduce approximately 60 kg of thionyl chloride, slowly and gradually. After stirring for a quarter of an hour, heat to reflux for 3 hours andthen evaporate off the ethanol under vacuum. Take up the residue with 300 liters of cyclohexane and heat to boiling. Allow to cool, filter, wash with cyclohexane and dry. 52.9 kg of ethyl L-norvalinate hydrochloride are obtained, that is a 97.6% yield.
Place 45 kg of ethyl N-norvalinate hydrochloride approximately 110 liters of water in a vessel equipped with a stirrer. Alkalify, then pour 23 kg of pyruvic acid very gradually into the solution obtained previously and stir the reaction mixture for 30 min. Place an aqueous suspension of charcoal containing 5% palladium and the alkaline solution of ethyl L-norvalinate obtained previously in a hydrogenation apparatus. Hydrogenate under pressure (30 bars) at room temperature for approximately one day. Filter under vacuum and evaporate the filtrate under reduced pressure, filter off and dry. Treat the residue obtained with ethanol; remove the insoluble material, consisting of sodium chloride, by filtration and rinse it with ethanol. Combine the ethanolic solutions; evaporate off the ethanol under reduced pressure and crystallize the residue from acetonitrile 34.3 kg of N-[(S)-1-carbethoxybutyl]-(S)-alanine are obtained, that is a 63.9% yield.
In a 30-liter reactor, reflux 12.5 kg of (2S,3aS,7aS)-2-carboxyperhydroindole, 50 kg of para-toluenesulfonic acid and 14.2 kg of benzyl alcohol and 38.4 kg of toluene, removing the water formed with the aid of a continuous separator. When no more water separates out, cool, filter off the precipitate of paratoluenesulfonate of the benzyl ester of (2S,3aS,7aS)-2carboxyoctahydroindole formed, and dry. Yield: 91.3%.
Add approximately 3.5 kg of triethylamine to a suspension of approximately 5 kg of para-toluenesulfonate of the benzyl ester of (2S,3aS,7aS)-2carboxyoctahydroindole in approximately 60 kg of ethyl acetate, followed by approximately 6 kg of 1-hydroxybenzotriazole, approximately 7.5 kg of the N[(S)-1-carbethoxybutyl]-(S)-alanine and approximately 7.0 kg of dicyclohexylcarbodiimide. Stir, cooling slightly for approximately 3 hours, then filter off the dicyclohexylurea formed and wash the organic phase with water. The dried organic phase is evaporated to dryness and benzyl ester of (2S,3aS,7aS)-1-{2-[1-(ethoxycarbonyl)-(S)-butylamino]-(S)propionyl}octahydroindole-2-carboxylic acid was obtained. Yield: 92.3%.
Dissolve, in a hydrogenator, 14 kg of benzyl ester of the (2S,3aS,7aS)-1-{2[1-(ethoxycarbonyl)-(S)-butylamino]-(S)-propionyl}octahydroindole-2carboxylic acid in cyclohexane. Add the charcoal containing 5% palladium and approximately 50 liters of water. Hydrogenate at ordinary temperature and pressure until the theoretical volume of hydrogen has been absorbed. Filter, wash the insoluble material with cyclohexane, separate off the organic phase and wash the aqueous phase again with cyclohexane. Isolate the (2S,3aS,7aS)-1-{2-[1-(ethoxycarbonyl)-(S)-butylamino]-(S)propionyl}octahydroindole-2-carboxylic acid from the aqueous phase by freeze-drying.
In practice it is used combined with 2-methyl-2-propanamine.
brand name
Aceon (Solvay Pharmaceuticals).
Therapeutic Function
Antihypertensive
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biochem/physiol Actions
Perindopril erbumine is an angiotensin converting enzyme (ACE) inhibitor; antihypertensive; becomes hydrolyzed in vivo to the active diacid metabolite; unlike the other ACE inhibitors, inhibits tumor growth in hepatocellular carcinoma cells due to suppression of VEGF levels; also suppresses angiotensin II production in vitro. Long-term therapy with this agent has a beneficial effect on the cerebral circulation by improving cerebral perfusion reserve in patients with previous minor stroke.
Safety Profile
A poison by intravenous route. Moderately toxic by ingestion, When heated to decomposition it emits toxic vapors of NOx.
Properties of Perindopril erbumine
| Melting point: | 126-128°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | powder |
| color | White |
Safety information for Perindopril erbumine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Perindopril erbumine
Perindopril erbumine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

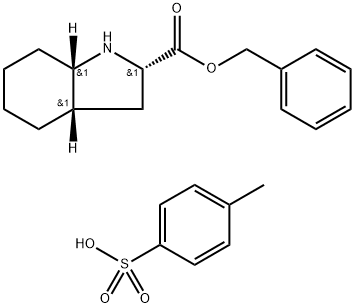
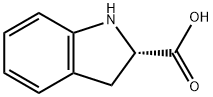

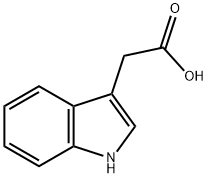
![N-[(S)-1-Carbethoxy-1-butyl]-(S)-alanine](https://img.chemicalbook.in/CAS/GIF/82834-12-6.gif)

You may like
-
 107133-36-8 Perindopril erbumine 98%View Details
107133-36-8 Perindopril erbumine 98%View Details
107133-36-8 -
 Perindopril erbumine 98%View Details
Perindopril erbumine 98%View Details
107133-36-8 -
 Perindopril erbumine 98.00% CAS 107133-36-8View Details
Perindopril erbumine 98.00% CAS 107133-36-8View Details
107133-36-8 -
 Perindopril erbumine CAS 107133-36-8View Details
Perindopril erbumine CAS 107133-36-8View Details
107133-36-8 -
 Perindopril erbumine CAS 107133-36-8View Details
Perindopril erbumine CAS 107133-36-8View Details
107133-36-8 -
 Perindopril erbumine CAS 107133-36-8View Details
Perindopril erbumine CAS 107133-36-8View Details
107133-36-8 -
 Perindopril erbumine CAS 107133-36-8View Details
Perindopril erbumine CAS 107133-36-8View Details
107133-36-8 -
 Coverheal Perindopril Erbumine 4mg TabletView Details
Coverheal Perindopril Erbumine 4mg TabletView Details
107133-36-8
