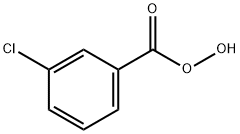
Perbenzoic acid
- CAS NO.:93-59-4
- Empirical Formula: C7H6O3
- Molecular Weight: 138.12
- MDL number: MFCD00216871
- EINECS: 202-260-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
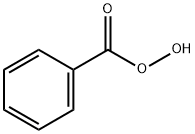
What is Perbenzoic acid?
Chemical properties
Volatile solid with a pungent odor; sublimes;mp 41–43°C (105.8–109°F); bp 100–105°C(212–221°F) at 15 torr (partially decomposes); slightly soluble in water but mixesreadily with most organic solvents.
The Uses of Perbenzoic acid
Perbenzoic acid is used to convert ethylenic compounds into oxides; in analysis of unsatd compounds, to determine the number of double bonds.
The Uses of Perbenzoic acid
Peroxybenzoic acid is used in organic analyses to measure the degree of unsaturation,and in making epoxides.
Synthesis Reference(s)
Tetrahedron, 23, p. 3327, 1967 DOI: 10.1016/S0040-4020(01)92300-2
Health Hazard
The toxicity of peroxybenzoic acid is verylow on animals. In humans it is almostnontoxic. Peroxybenzoic acid caused skintumor in mice on prolonged contact (NIOSH1986). Its tumorigenic action was lower thanthat of peroxyacetic acid. Its carcinogenicityin humans is unknown.
Fire Hazard
The presence of the peroxide functional group renders its strong oxidizing properties similar to those of other peroxy compounds. However, this compound is not hazardous like peroxyacetic or peroxyformic acids. It is not shock sensitive. Violent decomposition may occur only at high temperatures and/or in conjunction with certain organic contaminants that are easily oxidizable. Although there is no report of its explosion, general safety measures for handling peroxy compounds should be followed.
Safety Profile
Moderately irritating to skin, eyes, and mucous membranes by ingestion and inhalation. Questionable carcinogen with experimental tumorigenic data by skin contact. A dangerous fire hazard when exposed to heat, flame, or reducing materials. A powerful oxid
Purification Methods
Crystallise the peracid from *benzene or pet ether. It sublimes readily and is steam volatile. It is soluble in CHCl3, CCl4 and Et2O. [Braun Org Synth Coll Vol I 431 1941.] EXPLOSIVE.
Properties of Perbenzoic acid
| Melting point: | 41-43° |
| Boiling point: | bp15 100-110° (partial decomposition) |
| Density | 1.2667 (rough estimate) |
| refractive index | 1.4600 (estimate) |
| pka | 7.98±0.40(Predicted) |
| color | Leaflets or platelets from pet ether |
| CAS DataBase Reference | 93-59-4 |
Safety information for Perbenzoic acid
Computed Descriptors for Perbenzoic acid
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![BENZOYL PEROXIDE, [CARBONYL-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB2136130.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8