Minoxidil
Synonym(s):6-(1-Piperidinyl)-2,4-pyrimidinediamine 3-oxide;6-(1-Piperidinyl)pyrimidine-2,4-diamine 3-oxide;Minoxidil
- CAS NO.:38304-91-5
- Empirical Formula: C9H15N5O
- Molecular Weight: 209.25
- MDL number: MFCD00063409
- EINECS: 253-874-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04

What is Minoxidil?
Absorption
Minoxidil is at least 90% absorbed from the GI tract in experimental animals and man.
Toxicity
Oral LD50 in rats has ranged from 1321-3492 mg/kg; in mice, 2456-2648 mg/kg. Side effects include cardiovascular effects associated with hypotension such as sudden weight gain, rapid heart beat, faintness or dizziness.
Description
Minoxidil is a peripheral vasodilator that directly relaxes vascular smooth musculature, thus, lowering systolic and diastolic pressure. Its action is linked to the activation of calcium channels. Open calcium channels cause hyperpolarization of smooth muscle cells, which in turn, reduces the flow of calcium ions into the cell, which is necessary for supporting vascular tonicity. However, when taking minoxidil, tachycardia, elevated renin secretion, and water and sodium ion retention all appear simultaneously with hypotension.
Description
Minoxidil is the active ingredient in Rogaine, the popular hair growth product used by men and women suffering from hair loss. Originally marketed as a drug for high blood pressure, it was noted to have an interesting side effect--increased hair growth.
Chemical properties
White Crystalline Solid
Originator
Loniten ,Upjohn ,US ,1979
The Uses of Minoxidil
Used as an antihypertensive and antialopecia agent. Minoxidil activates ATP-activated K+ channels
The Uses of Minoxidil
A selective ATP dependent K+ (Kir6) channel activator
Background
Minoxidil is a potent direct-acting peripheral vasodilator (vasodilator agents) that reduces peripheral resistance and produces a fall in blood pressure.
Indications
For the treatment of severe hypertension and in the topical treatment (regrowth) of androgenic alopecia in males and females and stabilisation of hair loss in patients with androgenic alopecia.
What are the applications of Application
Minoxidil (U-10858) is a selective ATP dependent K+ (Kir6) channel activator
Definition
ChEBI: Minoxidil is a pyrimidine N-oxide that is pyrimidine-2,4-diamine 3-oxide substituted by a piperidin-1-yl group at position 6. It has a role as a vasodilator agent and an antihypertensive agent. It is a pyrimidine N-oxide, a member of piperidines and an aminopyrimidine.
Manufacturing Process
Barbituric acid is reacted with phosphorus oxychloride then with 2,4,6-trichloropyrimidine and that product with ammonia to give 4-chloro-2,6-diaminopyritnidine.
A 30 g (0.15 mol) quantity of 4-chloro-2,6-diaminopyrimidine is dissolved in600 ml of hot 3A alcohol, the solution cooled to 0°C to 10°C and 41.8 g (0.24mol) of m-chloroperbenzoic acid is added. The mixture is held at 0°C to 10°Cfor 4 hours and filtered. The solid is shaken for 2 hours in 0.24 mol of 10%sodium hydroxide and filtered. The solid is washed with water and dried toyield 193 g of crude product. This product is extracted for 1 hour with 900 mlof boiling acetonitrile to yield 14.8 g (44.7% yield) of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine, melting point 193°C.
A mixture of 3.0 g (0.019 mol) of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine and 35 ml of piperidine is refluxed for 1.5 hours, cooled andfiltered. The solid is shaken for 20 minutes in a solution of 0.8 g of sodiumhydroxide in 30 ml of water and filtered. The solid is washed with water andextracted with 800 ml of boiling acetonitrile and filtered to yield 3.5 g (89%)yield of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine, meltingpoint 248°C, decomposition at 259°C to 261°C.
brand name
Loniten (Pharmacia & Upjohn); Rogaine (Pharmacia & Upjohn); Minodyl (Quantum Pharmics).
Therapeutic Function
Antihypertensive
General Description
Minoxidil, 2,4-diamino-6-piperidinopyrimidine-3-oxide . It is converted to minoxidil sulfate in the liver bya sulfotransferase enzyme.
The antihypertensive properties of minoxidil are similarto those of hydralazine hydrochloride, in that minoxidil candecrease arteriolar vascular resistance. Minoxidil exerts itsvasodilatory action by a direct effect on arteriolar smoothmuscle and appears to have no effect on the CNS or on theadrenergic nervous system in animals. The serum half-lifeis 4.5 hours, and the antihypertensive effect may last up to24 hours.
Biological Activity
Antihypertensive. Antialopecia agent. K + channel (K ATP ) activator.
Biochem/physiol Actions
Activates ATP-activated K+ channels; vasodilator; slow or stop hair loss and promote hair regrowth.
Mechanism of action
Potassium channel openers are drugs that activate (i.e., open) ATP-sensitive K+
channels in the VSM. By
opening these potassium channels, there is increased efflux of potassium ions from the cells, causing hyperpolarization
of VSM, which closes the voltage-gated calcium channels and, thereby, decreases intracellular calcium. With less
calcium available to combine with calmodulin, there is less activation of MLCK and phosphorylation of myosin light
chains. This leads to relaxation and vasodilation. Because small arteries and arterioles normally have a high degree of
smooth muscle tone, these drugs are particularly effective in dilating these resistance vessels, decreasing systemic
vascular resistance, and lowering arterial pressure. The fall in arterial pressure leads to reflex cardiac stimulation
(baroreceptor-mediated tachycardia).
Minoxidil, as its active metabolite minoxidil O-sulfate, prolongs the opening of the potassium channel, sustaining
greater vasodilation on arterioles than on veins. The drug decreases blood pressure in both the supine and standing
positions, and there is no orthostatic hypotension. Associated with the decrease in peripheral resistance and blood
pressure is a reflex response that is accompanied by increased heart rate, cardiac output, and stroke volume, which
can be attenuated by the coadministration of a β-blocker. Along with this decrease in peripheral resistance is
increased plasma renin activity and sodium and water retention, which can result in expansion of fluid volume, edema,
and congestive heart failure. The sodium- and water-retaining effects of minoxidil can be reversed by coadministration
of a diuretic. When minoxidil is used in conjunction with a β-adrenergic blocker, pulmonary artery pressure remains
essentially unchanged.
Pharmacokinetics
Minoxidil is an orally effective direct acting peripheral vasodilator that reduces elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance. Minoxidil is also used topically to treat androgenetic alopecia. Microcirculatory blood flow in animals is enhanced or maintained in all systemic vascular beds. In man, forearm and renal vascular resistance decline; forearm blood flow increases while renal blood flow and glomerular filtration rate are preserved. The predominant site of minoxidil action is arterial. Venodilation does not occur with minoxidil; thus, postural hypotension is unusual with its administration. The antihypertensive activity of minoxidil is due to its sulphate metabolite, minoxidil sulfate.
Pharmacokinetics
Minoxidil is absorbed from the GI tract and is metabolized to its active sulfate metabolite. Plasma concentrations for minoxidil sulfate peak within 1 hour and then decline rapidly. Following an oral dose of minoxidil, its hypotensive effect begins in 30 minutes, is maximal in 2 to 8 hours, and persists for approximately 2 to 5 days. The delayed onset of the hypotensive effect for minoxidil is attributed to its metabolism to its active metabolite. The drug is not bound to plasma proteins. The major metabolite for minoxidil is its N-O-glucuronide, which unlike the sulfate metabolite is inactive as a hypotensive agent. Approximately 10 to 20% of an oral dose of minoxidil is metabolized to its active metabolite, minoxidil O-sulfate, and approximately 20% of minoxidil is excreted unchanged.
Clinical Use
Minoxidil is used for severe hypertension that is difficultto control with other antihypertensive agents. The drug hassome of the characteristic side effects of direct vasodilatorydrugs. It causes sodium and water retention and may requirecoadministration of a diuretic. Minoxidil also causes reflextachycardia, which can be controlled by use of a -adrenergicblocking agent.
Minoxidil topical solution is used to treat alopecia androgenitica(male-pattern baldness). Although the mechanismis not clearly understood, topical minoxidil is believed to increasecutaneous blood flow, which may stimulate hairgrowth. The stimulation of hair growth is attributed to vasodilationin the vicinity of application of the drug, resultingin better nourishment of the local hair follicles.
Side Effects
Adverse reactions include local irritation and contact dermatitis. If the treatment is discontinued, clinical regression occurs within 3 months to the state of hair thinning that would have occurred had the treatment not been started. Twice-daily treatment is more efficacious than once-daily application. Women who use the5%concentrationmay note the development of facial hair, which is reversible on discontinuation of the medication. The 5% concentration can be more irritating than the 2% solution.
Synthesis
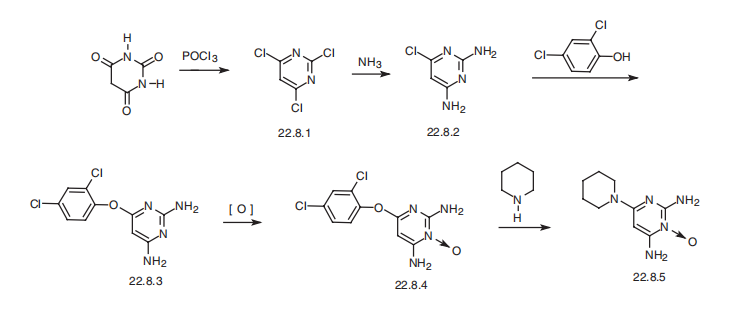
Minoxidil, 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidinopyrimidine (22.8.5), is synthesized from barbituric acid, the reaction of which with phosphorous oxychloride gives 2,4,6-trichloropyrimidine (22.8.1). Upon reaction with ammonium, this turns into 2,4-diamino-6-chloropyrimidine (22.8.2). Next, the resulting 2,4-diamino-6- chloropyrimidine (22.8.2) undergoes reaction with 2,4-dichlorophenol in the presence of potassium hydroxide, giving 2,4-diamino-6-(2,4-dichlorophenoxy)-pyrimidine (22.8.3). Oxidation of this product with 3-chloroperbenzoic acid gives 2,4-diamino-6-(2,4- dichlorophenoxy)pyrimidine-3-oxide (22.8.4), the 2,4-dichlorophenoxyl group of which is replaced with a piperidine group at high temperature, giving minoxidil (22.8.5).
Drug interactions
When minoxidil is administered with diuretics or other hypotensive drugs, the hypotensive effect of minoxidil increases, and concurrent use may cause profound orthostatic hypotensive effects.
Side Effects
Topical minoxidil is generally well tolerated, but it can cause temporary hair shedding, scalp irritation, and changes in hair texture. In rare cases, it can contribute to a fast heartbeat. Some minoxidil side effects can be avoided by taking the low-dose tablet formulation.
Metabolism
Approximately 90% of the administered drug is metabolized, predominantly by conjugation with glucuronic acid at the N-oxide position in the pyrimidine ring, but also by conversion to more polar products. Known metabolites exert much less pharmacologic effect than minoxidil itself.
Metabolism
Extensively metabolised by the liver. It requires sulphation to become active, but the major metabolite is a glucuronide conjugate. Excreted mainly in the urine in the form of metabolites. Minoxidil and its metabolites are dialysable, although the pharmacological effect is not reversed.
Storage
Room temperature
References
1) Meisheri et al. (1988), Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability; J. Pharmacol. Exp. Ther., 245 7 2) Messenger and Rundegren (2004) Minoxidil:mechanisms of action on hair growth; Br. J. Dermatol., 150 186
Properties of Minoxidil
| Melting point: | 272-274 °C (dec.) (lit.) |
| Boiling point: | 348.61°C (rough estimate) |
| Density | 1.1651 (rough estimate) |
| refractive index | 1.7610 (estimate) |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in water, soluble in methanol and in propylene glycol. |
| form | neat |
| pka | 4.61(at 25℃) |
| form | Solid |
| color | White |
| Water Solubility | Soluble in water (2.2 mg/ml), 100% ethanol (29 mg/ml), propylene glycol, acetone, DMSO (6.5 mg/ml), and methanol. |
| Merck | 14,6203 |
| Stability: | Stable for 2 years from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 38304-91-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Minoxidil(38304-91-5) |
Safety information for Minoxidil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Minoxidil
| InChIKey | ZFMITUMMTDLWHR-UHFFFAOYSA-N |
Minoxidil manufacturer
Kumar Organic Products Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






![MINOXIDIL, [PIPERYL-3,4-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB1362334.gif)
You may like
-
 Minoxidil 38304-91-5 98%View Details
Minoxidil 38304-91-5 98%View Details
38304-91-5 -
 Minoxidil 98% CAS 38304-91-5View Details
Minoxidil 98% CAS 38304-91-5View Details
38304-91-5 -
 Minoxidil Api Powder, Grade Standard: USPView Details
Minoxidil Api Powder, Grade Standard: USPView Details
38304-91-5 -
 Minoxidil Usp PowderView Details
Minoxidil Usp PowderView Details
38304-91-5 -
 Minoxidil Powder APIView Details
Minoxidil Powder APIView Details
38304-91-5 -
 Minoxidil Powder ApiView Details
Minoxidil Powder ApiView Details
38304-91-5 -
 Usp Minoxidil PowderView Details
Usp Minoxidil PowderView Details
38304-91-5 -
 Minoxidil (Pyrimidine Diamine Oxide) USP/EP/IP (Kopdil), 38304-91-5View Details
Minoxidil (Pyrimidine Diamine Oxide) USP/EP/IP (Kopdil), 38304-91-5View Details
38304-91-5
