pentorex
- CAS NO.:434-43-5
- Empirical Formula: C11H17N
- Molecular Weight: 163.26
- MDL number: MFCD01707973
- EINECS: 207-102-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-26 15:15:06
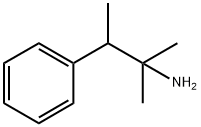
What is pentorex ?
Originator
Liprodene, Anphar-Rolland
Definition
ChEBI: Pentorex is a member of amphetamines.
Manufacturing Process
To a solution of 105.6 g of 2-phenylbutanone-3 in 110 ml ether was added dropwise a solution of methylmagnesium bromide (prepared from 19.4 g magnesium and 94.7 g methyl bromide in 400 ml of ether) for 60-90 min. Then the mixture was refluxed for 1 hour. After cooling to the mixture was added diluted sulfuric acid and then extracted with ether. Organic layer was dries with sodium sulfate. After evaporation of ether the 2-phenyl-3methylbutanol was distilled, B.P. 116-118°C/20 mm, yield 105 g, nd22 1.5152.
To 25.5 g of NaCN at 10-20°C ware added dropwise under stirring 64 ml of glacial acetic acid and ten at 20°C a mixture of 70 ml concentrated sulfuric acid and 64 ml of glacial acetic acid. To the prepared mixture at 20-25°C was added dropwise 82 g of 2-phenyl-3-methylbutanol. The mixture was stirred at 45-50°C for 10-20 min and then at 75°C for 30 min. To the reaction mixture was added 750 ml of water. The acids was neutralized with sodium carbonate. Product was extracted with ether and distilled. Boiling point of (dimethylbenzylcarbinyl)formamide 173-176°C/0 mm, yield 63 g.
52.3 g of (dimethylbenzylcarbinyl)formamide, 245 ml concentrated hydrochloric acid and 196 ml of water were refluxed for 6 hours. The unreacted compounds was extracted with ether. The residuum was stirred with sodium hydroxide and extracted with ether. By distillation was obtained 48.2 g of 2-amino-2-methyl-3-phenylbutane; B.P. 109-111°C/20 mm.
Hydrochloride of 2-amino-2-methyl-3-phenylbutane have melting point 164166°C.
In practice it is usually used as tartrate salt.
Therapeutic Function
Anorexic
Synthesis
Phenpentermine is prepared by Nformylating
the alcohol obtained from 3-phenyl-
2-butanone and methlymagnesium bromide; the
intermediate is hydrolyzed in acid .
Properties of pentorex
| Melting point: | 75-77 °C |
| Boiling point: | bp20 109-111° |
| Density | 0.926±0.06 g/cm3(Predicted) |
| pka | 10.00±0.25(Predicted) |
Safety information for pentorex
Computed Descriptors for pentorex
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1