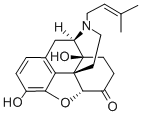
PENTAZOCINE
Synonym(s):(2R,6R,11R)-rel-1,2,3,4,5,6-Hexahydro- 6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol
- CAS NO.:359-83-1
- Empirical Formula: C19H27NO
- Molecular Weight: 285.43
- MDL number: MFCD00869259
- EINECS: 206-634-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is PENTAZOCINE?
Absorption
Well absorbed from the gastro-intestinal tract.
Chemical properties
Crystalline Solid
Originator
Pentazocine,Mallinckrodt Inc.
The Uses of PENTAZOCINE
(?)-Pentazocine is a benzomorphan-type opiate with analgesic properties. It binds to opioid receptors (Ki values = 7.2, 31, and 5.7 nM for κ-, δ-, μ-opioid receptors, respectively) and to the type 1 σ-receptor (Kd = 12 nM). (?)-Pentazocine is abused with methylphenidate and other drugs. It is regulated as a Schedule IV compound in the United States. This product is intended for forensic and research applications.
The Uses of PENTAZOCINE
Mixed opioid agonist-antagonist. Controlled substance. Analgesic (narcotic).
Indications
For the relief of moderate to severe pain.
Background
The first mixed agonist-antagonist analgesic to be marketed. It is an agonist at the kappa and sigma opioid receptors and has a weak antagonist action at the mu receptor. (From AMA Drug Evaluations Annual, 1991, p97)
Definition
ChEBI: Pentazocine is a benzazocine.
Manufacturing Process
A solution of 3,4-dimethylpyridine was added to a methyliodid. Then to the resulting solution containing 1,3,4-trimethylpyridinium iodide the 4methoxybenzylmagnesium chloride was added. After reaction process the 1,3,4-trimethyl-2-(4-methoxy-benzyl)-pyridine was obtained.
To the solution of 1,3,4-trimethyl-2-(4-methoxy-benzyl)-pyridine was reduced by hydrogen over 10% palladium-on-charcoal, and when reduction was complete, the catalyst was removed by filtration and the filtrate taken to dryness. The residue was recrystallized to give 2-(4-methoxybenzyl)-1,3,4trimethyl-1,2,5,6-tetrahydropyridine.
To the 2-(4-methoxybenzyl)-1,3,4-trimethyl-1,2,5,6-tetrahydropyridine the solution of hydrobromic acid was added and heated under reflux.The product obtained was recrystallized and yield N-methyl-1,2,3,4,5,6-hexahydro-6,11dimethyl-8-hydroxy-2,6-methano-3-benzazocine (2'-hydroxy-2,5,9trimethylbenzo-6-morphen), which then was demethylated by bromcyan (BrCN). As a result the racemic cis-1,2,3,4,5,6-hexahydro-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine was obtained (that is a. 2'-hydroxy-5,9dimethyl-6,7-benzomorphen).
A mixture of 8.7 g racemic cis-1,2,3,4,5,6-hexahydro-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine, 6.0 g of 1-bromo-3-methyl-2-butene, 5.0 g of sodium bicarbonate, and 125 ml of N,N-dimethylformamide was stirred and refluxed for approximately 4.5 hours. The reaction mixture was then filtered, and the solid on the filter was washed with ethanol. The filtrate and the wash liquor were combined, concentrated under reduced pressure, and then extracted with chloroform. The chloroform extract was concentrated under reduced pressure to yield a syrup which weighed 15.8 g. This syrup was dissolved in 120 ml of diethyl ether and the resulting solution was filtered to remove approximately 0.5 g of a brown amorphous solid. The filtrate was extracted with a mixture of 5 ml of concentrated hydrochloric acid and 20 ml of water. To the extract there was added 5 ml of concentrated ammonium hydroxide solution and ice. A pale tan syrup separated from solution and after stirring, this syrup solidified. The resulting pale tan solid was collected and dried; it weighed 10.6 g. After two recrystallizations from a mixture of methyl alcohol and water, with charcoaling, the 1,2,3,4,5,6-hexahydro-3-(3-methyl-2butenyl)-6,11-dimethyl-8-hydroxy-2,6-methano-3-benzazocine weighed 8.2 g and melted at 145°-147°C.
The1,2,3,4,5,6-hexahydro-3-(3-methyl-2-butenyl)-6,11-dimethyl-8-hydroxy2,6-methano-3-benzazocinewas soluble in a mixture of 0.35 ml of 2 N hydrochloric acid and 0.15 ml of water to the extent of 10%, the pH of the 1% solution being 2.80; and when the pH of the 1% solution was gradually raised by addition of 10 N sodium hydroxide solution, a precipitate formed at pH 5.4. The 1,2,3,4,5,6-hexahydro-3-(3-methyl-2-butenyl)-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine hydrochloride melted at 245°-247°C, dec.
brand name
Talwin (Sanofi Aventis);Fortagesic;Fortalgesic;Fortralin;Fortwin;Pentafen;Pentalgina;Talacen;Talwin nx.
Therapeutic Function
Analgesic
World Health Organization (WHO)
Pentazocine, which has both agonist and weak opioid antagonist activity, was introduced in 1967 for the treatment of moderate and severe pain. The risk of drug dependence is lower with pentazocine than with morphine-like drugs and pentazocine has been controlled under Section III of the 1971 Convention on Psychotropic Substances since 1984. The risk of dependence is now widely acknowledged to exist in vulnerable individuals and at least one country has applied controls analogous to those of Schedule I of the 1961 Single Convention on Narcotic Drugs. (Reference: (UNCPS3) United Nations Convention on Psychotropic Substances (III), , , 1971)
General Description
The benzomorphans are prepared synthetically and thus resultin several stereoisomers. The active benzomorphans arethose that have the equivalent bridgehead carbons in thesame absolute configuration of morphine (carbons 9, 13,and 14 of morphine). The only benzomorphan in clinical useis pentazocine, which is prepared as the 2(R), 6(R), 11(R)enantiomer (Chemical Abstracts numbering). Pentazocineis a mixed agonist/antagonist displaying differing intrinsicactivity at the opioid receptor subtypes. At the κ-receptor,pentazocine is a partial agonist and a weak antagonist.
According to the manufacturer, a 50-mg dose of pentazocinehas about the same analgesic potency as 60 mg of codeineand about 1/50th the antagonistic activity of nalorphine.74Pentazocine is also an agonist at the κ-receptor, and thismay be responsible for the higher percentage of patients thatexperience dysphoria with pentazocine versus morphine.75Some evidence also exists that women respond better to κ-agonists than men. Pentazocine is available in a 50-mgtablet along with a low dose of the antagonist naloxone 0.5mg (Talwin NX). Naloxone 0.5 mg orally is expected tohave no pharmacological effect but is included to dissuadeIV drug abusers from dissolving and injecting Talwin NX.
Pharmacokinetics
Pentazocine is a potent analgesic which when administered orally in a 50 mg dose appears equivalent in analgesic effect to 60 mg (1 grain) of codeine. Onset of significant analgesia usually occurs between 15 and 30 minutes after oral administration, and duration of action is usually three hours or longer. Onset and duration of action and the degree of pain relief are related both to dose and the severity of pretreatment pain. Pentazocine weakly antagonizes the analgesic effects of morphine and meperidine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Safety Profile
Poison by ingestion, subcutaneous, intramuscular, intraperitoneal, and intravenous routes. Experimental reproductive effects. Human systemic effects by intramuscular and intravenous routes: wakefulness, euphoria, hallucinations or distorted perceptions, tremors, convulsions, excitement, motor activity changes, muscle weakness, analgesia, withdrawal, parasympathomimetic effects, nausea or vomiting, and dernlititis. Can cause drug dependency and other central nervous system effects, An analgesic. When heated to decomposition it emits toxic fumes of NOx. See also ALLIT COMPOUNDS.
Metabolism
Hepatic
Properties of PENTAZOCINE
| Melting point: | 145.4-147.20C |
| Boiling point: | 427.85°C (rough estimate) |
| Density | 1.0104 (rough estimate) |
| refractive index | 1.5180 (estimate) |
| Flash point: | 9℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | DMSO: >10mg/mL |
| pka | pKa 8.68±0.05;11.23± 0.05(H2O,t =20,I=0.1) (Uncertain) |
| form | solid |
| color | white |
| CAS DataBase Reference | 359-83-1 |
| EPA Substance Registry System | 2,6-Methano-3-benzazocin-8-ol, 1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-buten-1-yl)-, (2R,6R,11R)-rel- (359-83-1) |
Safety information for PENTAZOCINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for PENTAZOCINE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6

![(+) PENTAZOCINE, [RING-1,3-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5473487.gif)