PENTACHLOROBENZENE
- CAS NO.:608-93-5
- Empirical Formula: C6HCl5
- Molecular Weight: 250.34
- MDL number: MFCD00000539
- EINECS: 210-172-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
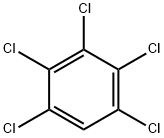
What is PENTACHLOROBENZENE?
Chemical properties
Pentachlorobenzene is a colorless crystalline solid. Pleasant aroma.
The Uses of PENTACHLOROBENZENE
It was used to prepare tetrachlorobenzene by photolysis.
The Uses of PENTACHLOROBENZENE
Agrochemical researc
Definition
ChEBI: A member of the class of pentachlorobenzenes that is benzene in which five of the hydrogens are replaced by chlorines. Now classed as a persistent organic pollutant under the Stockholm Convention.
Synthesis Reference(s)
The Journal of Organic Chemistry, 38, p. 2928, 1973 DOI: 10.1021/jo00957a002
General Description
White crystals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
PENTACHLOROBENZENE is relatively unreactive. May be incompatible with strong oxidizing and reducing agents. Also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Fire Hazard
Flash point data for PENTACHLOROBENZENE are not available but PENTACHLOROBENZENE is probably non-flammable.
Safety Profile
Moderately toxic by ingestion. An experimental teratogen. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC
Potential Exposure
Pentachlorobenzene is used primarily as a precursor in the synthesis of the fungicide pentachloronitrobenzene, and as a flame retardant. Drug/ Therapeutic Agent; Fungicide; bactericide; wood preservative; industrial Insecticides.
Source
Pentachlorobenzene may enter the environment from leaking dielectric fluids containing this compound. Pentachlorobenzene may be present as an undesirable by-product in the chemical manufacture of hexachlorobenzene, pentachloronitrobenzene, tetrachloroenzenes, tetrachloroethylene, trichloroethylene, and 1,2-dichloroethane (U.S. EPA, 1980).
Environmental Fate
Biological. In activated sludge, <0.1% mineralized to carbon dioxide after 5 days
(Freitag et al., 1985). From the first-order biotic and abiotic rate constants of pentachlorobenzene in estuarine water and sediment/water systems, the estimated biodegradation
half-lives were 4.6–6.5 and 6.0–7.6 days, respectively (Walker et al., 1988)
Photolytic. UV irradiation (λ = 2537 ?) of pentachlorobenzene in a hexane solution
for 3 hours produced a 50% yield of 1,2,4,5-tetrachlorobenzene and a 13% yield of 1,2,3,5-
tetrachlorobenzene (Crosby and Hamadmad, 1971). Irradiation (λ ≥285 nm)
A carbon dioxide yield of 2.0% was achieved when pentachlorobenzene adsorbed on
silica gel was irradiated with light (λ >290 nm) for 17 hours (Freitag et al., 1985).
The experimental first-order decay rate for pentachlorobenzene in an aqueous solution
containing a nonionic surfactant micelle (Brij 58, a polyoxyethylene cetyl ether) and
illuminated by a photoreactor equipped with 253.7-nm monochromatic UV lamps, is 1.47
× 10–2/sec. The corresponding half-life is 47 seconds. Photoproducts reported include, all
tetra-, tri- and dichlorobenzenes, chlorobenzene, benzene, phenol, hydrogen and chloride
ions (Chu and Jafvert, 1994)
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Polychlorinated hydrocarbons Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, aluminum, liquid oxygen; potassium, sodium.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of PENTACHLOROBENZENE
| Melting point: | 84-87 °C (lit.) |
| Boiling point: | 275-277 °C (lit.) |
| Density | 1.609 g/mL at 25 °C (lit.) |
| vapor pressure | 6 x 10-3 mmHg at 20–30 °C (quoted, Mercer et al., 1990) |
| refractive index | 1.5522 (estimate) |
| storage temp. | 2-8°C |
| solubility | Very soluble in ether (Sax and Lewis, 1987) |
| form | neat |
| color | White needles |
| Water Solubility | 1.332mg/L(25 ºC) |
| BRN | 1911550 |
| Henry's Law Constant | 5.14 at 25 °C (continuous flow sparger, Sproule et al., 1991) |
| CAS DataBase Reference | 608-93-5(CAS DataBase Reference) |
| EPA Substance Registry System | Pentachlorobenzene (608-93-5) |
Safety information for PENTACHLOROBENZENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for PENTACHLOROBENZENE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
