PENICILLIC ACID
- CAS NO.:90-65-3
- Empirical Formula: C8H10O4
- Molecular Weight: 170.16
- MDL number: MFCD00004365
- EINECS: 202-008-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
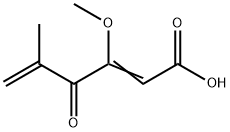
What is PENICILLIC ACID?
Description
Gram negative bacteria utilize N-
Chemical properties
white to light beige crystals or powder
The Uses of PENICILLIC ACID
Gram negative bacteria utilize N-acylated homoserine lactones to coordinate expression of virulence in response to the density of the surrounding bacterial population in a process termed quorum sensing. By interfering with this communication and thereby disrupting virulence expression, quorum sensing inhibitors are emerging as an alternative to the conventional ways of fighting bacterial infections. Penicillic acid is a mycotoxin produced from Penicillium species identified in molds on corn and other grains. At 80 μM, penicillic acid inhibits bacterial quorum sensing communication in P. aeruginosa by selectively repressing various virulence factor and other quorum sensing-regulated genes. It has been shown to inhibit Fas-mediated apoptosis at 100-200 μM by targeting caspase-8 activity in Burkitt’s lymphoma Raji cells.
The Uses of PENICILLIC ACID
Penicillic acid can be isolated from the seed-borne fungus Aspergillus persii EML-HPB1-11 and it exhibits antibacterial activity against various plant pathogenic bacteria. This compound can be a potential lead molecule for developing synthetic agrochemicals. Penicillic acid is also used as a quorum sensing inhibitor.
What are the applications of Application
Penicillic acid is an antibiotic mycotoxin
Safety Profile
Poison by intravenous, subcutaneous, and intraperitoneal routes. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic data. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
in vitro
the compound was active primarily against gram-negative bacteria, and it was also active against some gram-positive species. it showed limited antihelminthic properties but did affect the growth of oat seed coleoptiles by interference with the respiratory process. penicillic acid has proven to be too toxic for use in therapy [1]. penicillic acid (80 μm) inhibited bacterial quorum sensing communication in p. aeruginosa by selectively repressing various virulence factor and other quorum sensing-regulated genes [2]. in burkitt’s lymphoma raji cells, penicillic acid exihibited an inhibitory effect on fas-mediated apoptosis at 100-200 μm by targeting caspase-8 activity [3].
in vivo
the ldso (subcutaneous injection) for mice is 100 mg/kg. subcutaneous injection of 1.0 mg/dose twice weekly produced transplantable tumors after 64 weeks in all rats surviving treatment. in addition, a dose at 0.1 mg initiated tumor development [1].
Purification Methods
The lactone (furanone, anhydrous) hydrolyses to the acid (3-methoxy-4-oxo-hexa-2,5-dienoic acid, hydrate). It crystallises from water as the monohydrate (acid), or from pet ether as the anhydrous (lactone) compound. The free acid is in equilibrium with the lactone. UV: max 221nm ( 12,500) in 0.02M KOH; 228nm ( 11,500) in 0.02M HCl [Raphael J Chem Soc 1508 1948]. [Beilstein 3 II 519, 3 III 1467.] It is a possible antineoplastic.
References
[1] ciegler a, detroy r w, lillehoj e b. patulin, penicillic acid, and other carcinogenic lactones[j]. microbial toxins, 1971, 6: 409-434.
[2] rasmussen t b, skindersoe m e, bjarnsholt t, et al. identity and effects of quorum-sensing inhibitors produced by penicillium species[j]. microbiology, 2005, 151(5): 1325-1340.
[3] bando m, hasegawa m, tsuboi y, et al. the mycotoxin penicillic acid inhibits fas ligand-induced apoptosis by blocking self-processing of caspase-8 in death-inducing signaling complex[j]. journal of biological chemistry, 2003, 278(8): 5786-5793.
Properties of PENICILLIC ACID
| Melting point: | 83-87 °C |
| Boiling point: | 219.79°C (rough estimate) |
| Density | 1.1456 (rough estimate) |
| refractive index | 1.4425 (estimate) |
| storage temp. | Store at 2-8 |
| solubility | DMSO: >10mg/mL |
| form | solid |
| pka | 3.90±0.33(Predicted) |
| color | Needles from pet ether or rhombic orhexagonal plates |
| Merck | 13,7163 |
| CAS DataBase Reference | 90-65-3 |
| IARC | 3 (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Penicillic acid (90-65-3) |
Safety information for PENICILLIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for PENICILLIC ACID
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran



You may like
-
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
![2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
740842-50-6 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3