PAXILLINE
Synonym(s):
- CAS NO.:57186-25-1
- Empirical Formula: C27H33NO4
- Molecular Weight: 435.56
- MDL number: MFCD00083464
- EINECS: 637-206-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
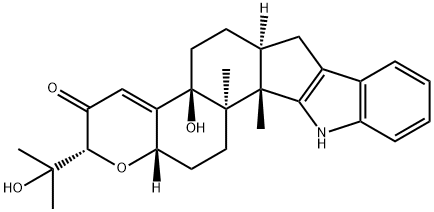
What is PAXILLINE?
Description
A complex alkaloid, paxilline occurs in the mycelium of the mold Penicillium paxilli. The structure has been confirmed by X-ray crystallography. The crystals are orthorhombic with space group P2 12 121 and a = 31.009, b = 11.522 and c = 7.70 A.
Description
Paxilline is an indole diterpene from fungi which potently and reversibly inhibits large conductance Ca2+-
Chemical properties
Powder
The Uses of PAXILLINE
Paxilline is a tremorgenic mycotoxin isolated from species of Penicillium, Acremonium and Emericella. Paxilline selectively blocks high-conductance Ca2+-activated potassium channels and inhibits binding to the cerebellar inositol 1,4,5-triphosphate (InsP(3)) receptor.
The Uses of PAXILLINE
Potent blocker of high-conductance calcium-activated potassium (BKCa) channels
What are the applications of Application
Paxilline is a selective and reversible calcium-activated potassium channel blocker
Definition
ChEBI: Paxilline is an indole diterpene alkaloid with formula C27H33NO4 isolated from Penicillium paxilli. It is a potent inhibitor of large conductance Ca2(+)- and voltage-activated K(+) (BK)-type channels. It has a role as a mycotoxin, a Penicillium metabolite, an anticonvulsant, an Aspergillus metabolite, a potassium channel blocker, a genotoxin, a geroprotector and an EC 3.6.3.8 (Ca(2+)-transporting ATPase) inhibitor. It is an organic heterohexacyclic compound, a tertiary alcohol, a terpenoid indole alkaloid, an enone and a diterpene alkaloid.
Biological Activity
Potent blocker of high-conductance Ca 2+ -activated K + (BK Ca ) channels. Binds to the α -subunit of BK Ca (K i = 1.9 nM for block of currents in α -subunit-expressing oocytes) and enhances binding of charybdotoxin to BK Ca channels in vascular smooth muscle. Also inhibits sarco/endoplasmic reticulum Ca 2+ -ATPase (IC 50 = 5-50 μ M).
Enzyme inhibitor
This BKCa/KCa1.1) channel blocker (FW = 435.56 g/mol; CAS 57186-25-1; Solubility: 100 mM in DMSO), also named (2R,4bS,6aS,12bS,12cR,14aS)- 5,6,6a,7,12,12b,12c,13,14,14a-decahydro-4b-hydroxy-2-(1-hydroxy-1- methylethyl)-12b,12c-dimethyl-2H-pyrano[2'',3'': 5',6']benz[1',2':6,7]indeno [1,2-b]indol-3(4bH)-one, binds to the α-subunit of BKCa, exhibiting a Ki value of 1.9 nM in blocking currents in α-subunit-expressing oocytes and enhancing binding of to BKCa channels in vascular smooth muscle. (See Charybdotoxin) Paxilline also inhibits sarco/endoplasmic reticulum Ca2+-ATPase, IC50 = 5 - 50 μM.
storage
-20°C (desiccate)
References
Springer et ai., Tetrahedron Lett., 2531 (1975)
Properties of PAXILLINE
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO, acetone or chloroform. |
| form | powder |
| color | faint yellow |
Safety information for PAXILLINE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PAXILLINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Paxilline CAS 57186-25-1View Details
Paxilline CAS 57186-25-1View Details
57186-25-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1