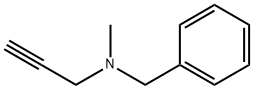
PARGYLINE HYDROCHLORIDE
Synonym(s):N-Methyl-N-(2-propynyl)benzylamine hydrochloride;N-Methyl-N-propargylbenzylamine hydrochloride
- CAS NO.:306-07-0
- Empirical Formula: C11H14ClN
- Molecular Weight: 195.69
- MDL number: MFCD00012492
- EINECS: 206-175-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
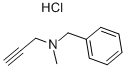
What is PARGYLINE HYDROCHLORIDE?
Originator
Eutonyl, Abbott , US ,1963
The Uses of PARGYLINE HYDROCHLORIDE
Pargyline hydrochloride has been used:
- as a histone demethylase inhibitor to treat monocytes to investigate whether various inhibitors affect training
- to maximize uptake into dopaminergic neurons (DA) neurons in experimental rats,
- to study its effect on discolouration of medullary tissues of sclerotia of S. sclerotiorum
The Uses of PARGYLINE HYDROCHLORIDE
Antihypertensor;Monoamine oxidase inhibitor
What are the applications of Application
Pargyline hydrochloride is an irreversible MAO-B inhibitor
Manufacturing Process
A mixture of 23.8 grams (0.2 mol) of propargyl bromide, 24.2 grams (0.2 mol) of N-methylbenzylamine and 400 ml of anhydrous ethanol in the presence of 42.4 grams (0.4 mol) of anhydrous sodium carbonate was heated at the boiling temperature and under reflux for a period of 17 hours.
The sodium carbonate was then removed by filtration and the alcohol was removed by distillation under reduced pressure. The residue was treated with 300 ml of dry ether and the resulting solution was filtered to remove sodium bromide.
The filtrate was dried and fractionally distilled under reduced pressure to obtain the desired N-methyl-N-propargylbenzylamine which boiled at 96°97°C at 11 mm pressure.
Analysis calculated for C11H13N: C = 82.97%; H = 8.23%; N = 8.80%. Found: C = 82.71%; H = 8.51%; N = 8.93%.
The hydrochloride salt of this amine was prepared by dissolving the amine in ether and adding ethereal hydrogen chloride to the ether solution. The solid hydrochloride salt which precipitated was recrystallized from an ethanol-ether mixture and was found to melt at 154° - 155°C.
brand name
Eutonyl (Abbott).
Therapeutic Function
Antihypertensive
Biological Activity
pargyline, an irreversible and non-selective inhibitor of mao, is used to treat moderate hypertension and cancer cells. there are two isoforms of mao, mao-a and mao-b. mao-a preferentially deaminates melatonin, serotonin, norepinephrine and epinephrine. mao-b catalyzes the oxidative deamination and inactivation of certain catecholamines within the presynaptic nerve terminals.
Biochem/physiol Actions
Pargyline hydrochloride acts as a histone demethylase inhibitor. It has antihypertensive action.
Safety Profile
Poison by ingestion,intraperitoneal, and intravenous routes. Human systemiceffects by ingestion: effects on fluid intake, psychologicaleffects. Experimental reproductive effects. When heatedto decomposition it emits very toxic fumes of HCl andNOx.
in vitro
pargyline reduced the proliferation of human prostate carcinoma (lncap-ln3) cells in a time- and dose-dependent fashion. in addition, compared to the control, pargyline remarkably triggered cell cycle arrest at the g1 phase. pargyline elicited an increase in the cell death rate via promoting apoptosis, suggesting that pargyline was a powerful candidate drug for the treatment of human prostate cancer [1].
in vivo
male rats were injected intraperitoneally pargyline at a dose of 75 mg/kg for 80 min. pargyline significantly increased the concentration of extracellular dopamine in the striatum and simultaneously, significantly reduced the concentration of extracellular mao-derived metabolite 3,4-dihydroxyphenylacetic acid to undetectable levels [2].
References
[1]. chai, y. effects of the monoamine oxidase inhibitors pargyline and tranylcypromine on cellular proliferation in human prostate cancer cells. oncology reports. 2013.
[2]. desvignes, c., bert, l., vinet, l., denoroy, l., renaud, b., & lambás-seas, l. evidence that the neuronal nitric oxide synthase inhibitor 7-nitroindazole inhibits monoamine oxidase in the rat: in vivo effects on extracellular striatal dopamine and 3,4-dihydroxyphenylacetic acid. neuroscience letters. 1999; 261(3):175-178.
Properties of PARGYLINE HYDROCHLORIDE
| Melting point: | 160-163 °C(lit.) |
| storage temp. | -20°C |
| solubility | ≥33.55 mg/mL in EtOH; ≥51.6 mg/mL in H2O; ≥7.55 mg/mL in DMSO |
| form | A crystalline solid |
| color | Crystals from EtOH/Et2O |
| Merck | 13,7110 |
| BRN | 3699487 |
| CAS DataBase Reference | 306-07-0 |
Safety information for PARGYLINE HYDROCHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for PARGYLINE HYDROCHLORIDE
| InChIKey | BCXCABRDBBWWGY-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Pargyline hydrochloride >95% CAS 306-07-0View Details
Pargyline hydrochloride >95% CAS 306-07-0View Details
306-07-0 -
 Pargyline hydrochloride CAS 306-07-0View Details
Pargyline hydrochloride CAS 306-07-0View Details
306-07-0 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4