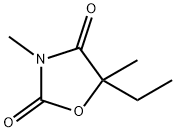
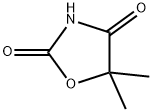
PARAMETHADIONE (500 MG)
- CAS NO.:115-67-3
- Empirical Formula: C7H11NO3
- Molecular Weight: 157.17
- MDL number: MFCD00865309
- EINECS: 2040988
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:31
What is PARAMETHADIONE (500 MG)?
Absorption
Rapid via the digestive tract.
Toxicity
Symptoms of overdose include clumsiness or unsteadiness, coma, severe dizziness, severe drowsiness, severe nausea, and problems with vision.
Originator
Paradione,Abbott, US ,1949
The Uses of PARAMETHADIONE (500 MG)
Paramethadione is also used in minor forms of epilepsy.
The Uses of PARAMETHADIONE (500 MG)
Paramethadione acts as an antiepileptic and anticonvulsant agent.
Background
Paramethadione is an anticonvulsant in the oxazolidinedione class. It is associated with fetal trimethadione syndrome, which is also known as paramethadione syndrome.
Indications
Used for the control of absence (petit mal) seizures that are refractory to treatment with other medications.
Definition
ChEBI: Paramethadione is an oxazolidinone.
Manufacturing Process
About 143.1 grams (one mol) of 5-methyl-5-ethyloxazolidine-2,4-dione is dissolved in 300 cc of methanol containing 23 grams of sodium. To the above mixture is added 126 grams of dimethyl sulfate in 10 cc portions while the temperature is maintained at about 50°C by external cooling. The mixture is then heated briefly to boiling, cooled, diluted with about 500 cc of water and extracted with two 250 cc portions of benzene. The benzene extract is separated, washed once with sodium bicarbonate solution and once with water. The benzene is removed by evaporation on a steam bath and the residue is fractionally distilled. The material boiling at 112° to 116°C at 25 mm pressure is taken; nD25=1.4495. Upon further fractionation, a very pure specimen boils at 101°-102°C at 11 mm.
The 5-methyl-5-ethyloxazolidine-2,4-dionemay be prepared by reacting methyl ethyl ketone with sodium cyanide and with ammonium thiocyanate followed by desulfurization. This intermediate may also be prepared by condensing α-hydroxy-α-methylbutyramide with ethyl chlorocarbonate or by condensing ethyl α-hydroxy-α-methylbutyrate with urea. Another method described (Traube and Aschar, Ber., 46, 2077-1913) consists in the condensation of ethyl α-hydroxy-α-methylbutyrate with guanidine followed by hydrolysis.
brand name
Paradione (Abbott).
Therapeutic Function
Anticonvulsant
Pharmacokinetics
Paramethadione is an oxazolidinedione anticonvulsant similar to trimethadione that acts on the central nervous system (CNS) to reduce the number of absence seizures (often seen in epileptics). Absence seizures involve an interruption to consciousness where the person experiencing the seizure seems to become vacant and unresponsive for a short period of time (usually up to 30 seconds). Paramethadione acts on thalamic neurons in the thalamic reticular nucleus (which studies have shown to be associated with absence seizures, von Krosigk et al., 1993).
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Experimental teratogenic effects. Other experimental reproductive effects. Whenheated to decomposition it emits toxic fumes of NOx.
Synthesis
Paramethadione, 5-ethyl-3,5-dimethyloxazolidine-2,4-dione (9.8.3), differs from trimethadione only in the substitution of one methyl group with an ethyl group. It is synthesized in a completely analogous manner, except that it comes from 2-hydroxy-2-methylbutyric acid instead of 2-hydroxyisobutyric acid [29].
Metabolism
Primarily hepatic (mainly via cytochrome P450 isozyme 2C9), paramethadione is completely demethylated to 5-ethyl-5-methyl-2,4-oxazolidinedione, the active metabolite.
Properties of PARAMETHADIONE (500 MG)
| Melting point: | 31-32 °C |
| Boiling point: | 281.82°C (rough estimate) |
| Density | d425 1.1180-1.1240 |
| refractive index | nD25 1.449 |
| pka | -2.18±0.40(Predicted) |
| EPA Substance Registry System | Paramethadione (115-67-3) |
Safety information for PARAMETHADIONE (500 MG)
Computed Descriptors for PARAMETHADIONE (500 MG)
Abamectin manufacturer
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![3-(2-AMINO-ETHYL)-1-OXA-3-AZA-SPIRO[4.4]NONANE-2,4-DIONE HYDROCHLORIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure9/GIF/CB9137338.gif)

You may like
-
 115-67-3 99%View Details
115-67-3 99%View Details
115-67-3 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4