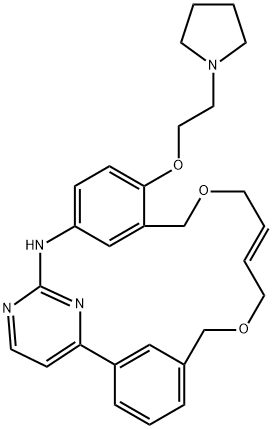
PACRITINIB |CAS 937272-79-2
- CAS NO.:937272-79-2
- Empirical Formula: C28H32N4O3
- Molecular Weight: 472.58
- MDL number: MFCD22572772
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
What is PACRITINIB |CAS 937272-79-2?
Absorption
Following oral administration of 200mg pacritinib twice daily, the mean Cmax and AUC0-12 at steady-state were 8.4 mg/L and 95.6 mg*h/L, respectively. The Tmax is approximately 4-5 hours post-dose. Co-administration with food does not significantly impact the pharmacokinetics of pacritinib.
Toxicity
Overdosage with pacritinib may lead to gastrointestinal toxicity, myelosuppression, blurred vision, dizziness, worsening performance status, and sepsis. There is no known antidote for pacritinib overdose, and hemodialysis is not expected to enhance its elimination.
Description
Pacritinib received accelerated approval in 2022 for the treatment of adults with intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with a platelet count below 50 × 109 /L, which affects 26% of all myelofibrosis patients (~20,000 in the United States). Unlike several other JAK2 inhibitors, pacritinib exhibits good selectivity (~60-fold) for JAK2 over JAK1 while also inhibiting JAK3 with about 25-fold lower potency than JAK2. Pacritinib exhibits equipotent activity against FLT3 (IC50 = 22 nM) and mutant FLT3-D835Y (IC50 = 6?nM) at the same potency range as JAK2 (JAK2 IC50 = 23 nM).
Characteristics
Class: non-receptor tyrosine kinase (JAK3)
Treatment: myelofibrosis
Elimination half-life = 40 h
Protein binding = 99%
The Uses of PACRITINIB |CAS 937272-79-2
Pacritinib is a JAK2/FLT3 inhibitor used in the treatment of acute myeloid leukemia showing significant tumor growth inhibition and lung metastasis.
Background
Myelofibrosis (MF) is a rare disorder characterized by hematopoietic abnormalities and fibrosis within the bone marrow. The underlying cause of primary MF is unknown, but secondary MF can arise in patients with a history of polycythemia vera or essential thrombocythemia. While some patients may remain asymptomatic, typical symptoms of MF arise from abnormalities in blood cell production and may therefore include various cytopenias, infections, splenomegaly, and general systemic symptoms such as fever. Approximately 50% of patients with primary MF have a mutation of the JAK2 gene, which is also commonly mutated in patients with polycythemia vera or essential thrombocythemia. JAK2 signaling is important for hematopoiesis and proper immune functioning, and while the precise role it plays in the pathogenesis of MF remains unclear, its clear association with MF has made it a desirable therapeutic target in MF treatment.
Pacritinib is an inhibitor of both wild-type and mutant (V617F) JAK2, as well as FMS-like tyrosine kinase 3 (FLT3), which was granted accelerated approval by the FDA in February 2022 for the treatment of both primary and secondary MF in patients with platelet counts < 50 x 109/L. It provides a treatment option for patients who have MF with severe thrombocytopenia, which occurs in approximately one-third of MF patients and carries with it a particularly poor prognosis.
Indications
Pacritinib is indicated for the treatment of adults with intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with a platelet count below 50 x 109/L.
This indication is approved under accelerated approval based on spleen volume reduction. Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
Mechanism of action
Pacritinib competes with JAK2 for ATP binding, which may result in inhibition of JAK2 activation, inhibition of the JAK-STAT signaling pathway, and so caspase-dependent apoptosis.
Pharmacokinetics
Pacritinib is administered orally twice daily, with or without food. It should not be used in patients with moderate or severe (Child-Pugh B or C) hepatic impairment, nor in patients with significant renal impairment (eGFR <30 mL/min).
Patients taking pacritinib may experience a prolonged QTc interval - while no cases of torsades de pointes have been reported in clinical trials, QTc prolongations to >500 msec and/or increases in baseline QTc by >60 msec were observed in some patients during clinical trials. A baseline QTc interval should be obtained prior to initiating therapy and regular monitoring (including for risk factors, e.g. hypokalemia) should continue throughout therapy.
Enzyme inhibitor
This pyrimidine-based macrocycle and potent protein kinase inhibitor (FW = 472.59 g/mol; CAS 937272-79-2; Solubility: 11 mg/mL DMSO), also known as SB1518, selectively targets Janus Kinase 2 (or JAK2; IC50 = 23 nM) and Fms-Like Tyrosine Kinase-3 (or FLT3; IC50 = 22 nM), with values of 1280 and 522 nM for JAK1 and JAK3. FLT3 is genetically altered in up to 35% of acute myeloblastic leukemias, making it an attractive target for AML patients. Pacritinib has potent anti-proliferative effects on myeloid and lymphoid cell lines driven by mutant or wild-type JAK2 or FLT3, resulting from cell cycle arrest and induction of apoptosis. SB1518 has favorable pharmacokinetic properties after oral dosing in mice, is well tolerated and significantly reduces splenomegaly and hepatomegaly in a JAK2(Val-617-Phe)-driven disease model. Primary Mode of Inhibitory Action: Upregulation of JAK2 in FLT3-TKI-resistant AML cells was identified as a potential mechanism of resistance to selective FLT3 inhibition. This resistance could be overcome by pacritinib’s combined inhibition of FLT3 and JAK2 in this cellular model. Pacritinib potently inhibits FLT3 auto-phosphorylation and downstream STAT5, MAPK and PI3K signaling pathways in AML cell lines with highest potency against cells harboring FLT3-ITD mutations. Blockade of FLT3 signaling was also demonstrated in primary AML blasts treated ex vivo with pacritinib. In both cell lines and primary blasts, pacritinib treatment led to the induction of G1 arrest, inhibition of cell proliferation, as well as caspase-dependent apoptosis. The anti-proliferative effects of pacritinib on the FLT3-ITD harboring cell lines MV4-11 (IC50 = 47 nM) and MOLM-13 (IC50 = 67 nM), which have been reported previously,16 are in the same range as the inhibition of intracellular FLT3 signalling.
Metabolism
Pacritinib metabolism is mediated primarily by CYP3A4. While it undergoes extensive metabolism to at least four identified metabolites - M1, M2, M3, and M4 - parent drug is the major circulating component in plasma and is responsible for the pharmacologic activity. The two major metabolites, M1 and M2, represent 9.6% and 10.5% of parent drug exposure, respectively.
Properties of PACRITINIB |CAS 937272-79-2
| Boiling point: | 694.3±65.0 °C(Predicted) |
| Density | 1.22±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:5.0(Max Conc. mg/mL);10.6(Max Conc. mM) |
| form | A crystalline solid |
| pka | 9.56±0.20(Predicted) |
| color | Light yellow to yellow |
Safety information for PACRITINIB |CAS 937272-79-2
Computed Descriptors for PACRITINIB |CAS 937272-79-2
PACRITINIB |CAS 937272-79-2 manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 937272-79-2 Pacritinib 98%View Details
937272-79-2 Pacritinib 98%View Details
937272-79-2 -
 Pacritinib 98+View Details
Pacritinib 98+View Details
937272-79-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8