p-Tolunitrile
Synonym(s):4-Methylbenzonitrile
- CAS NO.:104-85-8
- Empirical Formula: C8H7N
- Molecular Weight: 117.15
- MDL number: MFCD00001827
- EINECS: 203-244-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
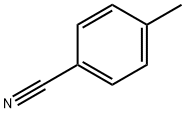
What is p-Tolunitrile?
Chemical properties
This substance is a clear colorless liquid or beige solid that exhibits toxicity and flammability. It should avoid contact with the skin.
The Uses of p-Tolunitrile
p-?Tolunitrile is a reagent used in the preparation of 1H-Indazoles from imidates and nitrosobenzenes.
Synthesis Reference(s)
Synthetic Communications, 15, p. 1299, 1985 DOI: 10.1080/00397918508077278
Tetrahedron Letters, 27, p. 1925, 1986 DOI: 10.1016/S0040-4039(00)84413-5
General Description
Beige solid at 62.6° F.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Nitriles, such as p-Tolunitrile, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
Fire Hazard
p-Tolunitrile is combustible.
Purification Methods
Melt the nitrile, dry it with MgSO4, fractionally crystallise it from its melt, then fractionally distil it under reduced pressure in a 6-in spinning band column. [Brown J Am Chem Soc 81 3232 1959.] It can also be crystallised from *benzene/pet ether (b 40-60o). [Beilstein 9 H 489, 9 I 194, 9 II 330, 9 III 2348, 9 IV 1738.]
Properties of p-Tolunitrile
| Melting point: | 26-28 °C(lit.) |
| Boiling point: | 103-106 °C20 mm Hg(lit.) |
| Density | 0.981 g/mL at 25 °C(lit.) |
| refractive index | 1.5285-1.5305 |
| Flash point: | 185 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: very soluble |
| form | Viscous Liquid |
| color | Clear |
| Specific Gravity | 0.981 |
| Water Solubility | <0.1 g/100 mL at 17 ºC |
| Merck | 14,9538 |
| BRN | 507386 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 104-85-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzonitrile, 4-methyl-(104-85-8) |
| EPA Substance Registry System | p-Tolunitrile (104-85-8) |
Safety information for p-Tolunitrile
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for p-Tolunitrile
| InChIKey | VCZNNAKNUVJVGX-UHFFFAOYSA-N |
p-Tolunitrile manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 104-85-8 98%View Details
104-85-8 98%View Details
104-85-8 -
 p-Tolunitrile, 98% CAS 104-85-8View Details
p-Tolunitrile, 98% CAS 104-85-8View Details
104-85-8 -
 4-Methyl benzonitrile CAS 104-85-8View Details
4-Methyl benzonitrile CAS 104-85-8View Details
104-85-8 -
 p-Tolunitrile CAS 104-85-8View Details
p-Tolunitrile CAS 104-85-8View Details
104-85-8 -
 P-Tolunitrile 98% CAS 104-85-8View Details
P-Tolunitrile 98% CAS 104-85-8View Details
104-85-8 -
 p-Tolunitrile 95.00% CAS 104-85-8View Details
p-Tolunitrile 95.00% CAS 104-85-8View Details
104-85-8 -
 p-Tolunitrile 98% (GC) CAS 104-85-8View Details
p-Tolunitrile 98% (GC) CAS 104-85-8View Details
104-85-8 -
 4-TOLUNITRILEView Details
4-TOLUNITRILEView Details
104-85-8
