p-Toluenesulfonic acid
- CAS NO.:104-15-4
- Empirical Formula: C7H8O3S
- Molecular Weight: 172.2
- MDL number: MFCD00064387
- EINECS: 203-180-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-24 20:53:42
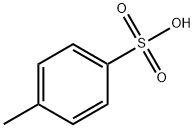
What is p-Toluenesulfonic acid?
Description
p-Toluene sulfonic acid (PTSA) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.
TsOH is a strong organic acid, about a million times stronger than benzoic acid. It is one of the few strong acids that is solid and, hence, conveniently weighed. Also, unlike some strong mineral acids (especially nitric acid, sulfuric acid, and per chloric acid), TsOH is non - oxidizing.
Chemical properties
Clear colorless to light yellow solution
Chemical properties
Decomposes on heating and on burning. p-Toluenesulfonic acid produces toxic and corrosive fumes including sulfur oxides. It is a strong acid. It reacts violently with bases and is corrosive. Attacks many metals.
Reactions
TsOH may be converted to p-toluenesulfonic anhydride by heating with phosphorus pentoxide.
When heated with acid and water, TsOH undergoes hydrolysis to toluene:
CH3C6H4SO3H + H2O → C6H5CH3 + H2SO4
The Uses of p-Toluenesulfonic acid
p-Toluenesulfonic Acid Hydrate (Lisinopril EP Impurity B) is used in the synthesis of resveratrol. Also used in the synthesis of oxane derivatives as antimalarial agents.
Definition
ChEBI: p-Toluenesulfonic acid is an arenesulfonic acid that is benzenesulfonic acid in which the hydrogen at position 4 is replaced by a methyl group. It is a member of toluenes and an arenesulfonic acid. It is a conjugate acid of a toluene-4-sulfonate.
Reactions
p-Toluene sulfonic acid may be converted to p-toluene sulfonic anhydride by heating with phosphorus pentoxide.
When TsOH is heated with acid and water, a hydrolysis reaction takes place and toluene is formed:
CH3C6H4SO3H + H2O → C6H5CH3 + H2SO4
This reaction is general for aryl sulfonic acids, but the rate at which it occurs depends upon the structure of the acid, the temperature and the nature of the catalyzing acid. For example p- TsOH is unaffected by cold concentrated hydrochloric acid, but hydrolyzes when heated to 186°C in concentrated phosphoric acid.
Preparation
P-Toluenesulfonic acid is prepared on an industrial scale by the sulfonation of toluene.
Health hazards
p-Toluenesulfonic acid is corrosive to the eyes, skin and respiratory tract. Corrosive on ingestion. Inhalation of the aerosol may cause lung oedema. See Notes. Medical observation is indicated.
Flammability and Explosibility
Non flammable
Preparation and handling
TsOH is prepared on an industrial scale by the sulfonation of toluene. It hydrates readily. Common impurities include benzene sulfonic acid and sulfuric acid. Impurities can be removed by recrystallization from its concentrated aqueous solution followed by azeotropic drying with toluene.
Toluene sulfonic acid finds use in organic synthesis as an "organic - soluble" acid catalyst. Examples of uses :
Acetalization of an aldehyde.
Esterification of carboxylic acids.
Trans esterification of an ester.
Tosylate esters
Tosylate esters are used as alkylating agents because the tosyl group is electron-with drawing, which makes the tosylate anion a good leaving group. The tosyl group is also a protecting group for alcohols and amines, prepared by combining the alcohol with 4- toluenesulfonyl chloride, usually in an aprotic solvent, often pyridine, the basicity of which activates the reaction. Toluenesulfonate esters undergo nucleophilic attack or elimination. Reduction of tosylate esters gives the hydrocarbon. Thus, tosylation followed by reduction allows for the deoxygenation of alcohols.
Properties of p-Toluenesulfonic acid
| Melting point: | 106~107℃ |
| Boiling point: | 116 °C |
| Density | 1.07 |
| vapor pressure | 69.8Pa at 20℃ |
| refractive index | 1.3825-1.3845 |
| Flash point: | 41 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | -0.43±0.50(Predicted) |
| form | Solution |
| color | Clear colorless to light yellow |
| Odor | pungent odor |
| Water Solubility | soluble |
| CAS DataBase Reference | 104-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | P-toluene sulfonic acid(104-15-4) |
| EPA Substance Registry System | 4-Methylbenzenesulfonic acid (104-15-4) |
Safety information for p-Toluenesulfonic acid
Computed Descriptors for p-Toluenesulfonic acid
p-Toluenesulfonic acid manufacturer
C.H. Chemicals
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 104-15-4 PARA TOLUENE SULFONIC ACID( PTSA) 99%View Details
104-15-4 PARA TOLUENE SULFONIC ACID( PTSA) 99%View Details
104-15-4 -
 Para Toluene Sulphonic Acid 99%View Details
Para Toluene Sulphonic Acid 99%View Details -
 4-Toluenesulfonic acid 98%View Details
4-Toluenesulfonic acid 98%View Details -
 104-15-4 4-Toluenesulfonic acid 98%View Details
104-15-4 4-Toluenesulfonic acid 98%View Details
104-15-4 -
 104-15-4 4-Toluenesulfonic acid 98%View Details
104-15-4 4-Toluenesulfonic acid 98%View Details
104-15-4 -
 PTSA CASView Details
PTSA CASView Details -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3
