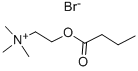
oxypyrronium bromide
- CAS NO.:561-43-3
- Empirical Formula: C21H32BrNO3
- Molecular Weight: 426.38768
- MDL number: MFCD00865217
- EINECS: 2092198
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
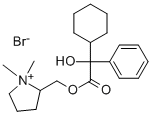
What is oxypyrronium bromide?
Originator
Oxypyrronium ,Shanghai Lansheng
Manufacturing Process
The 1-methyl-2-hydroxymethylpyrrolidine was obtained by the process of Application No 21193/56 (Serial No. 820,503).
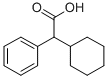
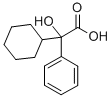
A methanolic solution of sodium methoxide [from sodium (0.6 g) and methanol (15 ml)] was added dropwise during 3 h to a boiling solution of methyl phenylcyclohexylglycollate (33.7 g) and 1-methyl-2hydroxymethylpyrrolidine (23.4 g) in heptane (400 ml) and the methanol that separated was removed by means of a Dean and Stark apparatus. At the end of 4 h no further separation of methanol occurred and the solvent was removed under reduced pressure. The residue was dissolved in either and the etheral solution, after washing with water (3 x 50 ml), was extracted with 5 N hydrochloric acid (3 x 100 ml). The (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (35.5 g 71%) crystallised out of the acid extract as colourless needles, melting point 181°-196°C. Extraction of this hydrochloride (33.0 g) with hot ethanol (150 ml) left the sparingly soluble (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (aform) (7.6 g), melting point 220°-222°C.
The (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (aform) (15.0 g) was dissolved in water, basified with sodium hydroxide solution and the resultant oil extracted into ether. The extracts were dried over magnesium sulfate, the ether evaporated and the residue dissolved in acetone (100 ml). Methyl bromide (7.8 g, 2 mole) was added to the acetone solution and the mixture warmed on a steam bath for 15 min. The solution was cooled and the solid filtered off, washed with a little acetone and dried to give the
(1,1-dimethyl-2-pyrrolidyl)methyl α-phenylcyclokexylglycollate bromide, melting point 185°-186°C. (86%).
Therapeutic Function
Anticholinergic, Spasmolytic
Safety information for oxypyrronium bromide
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole 4-Ethylbenzylamine N-(5-Amino-2-methylphenyl)acetamide 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazoleRelated products of tetrahydrofuran


You may like
-
 100-71-0 99%View Details
100-71-0 99%View Details
100-71-0 -
 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
127464-43-1 -
 13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 -
 1446013-08-6 98%View Details
1446013-08-6 98%View Details
1446013-08-6 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3