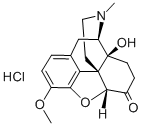
OXYMORPHONE
Synonym(s):4,5α-Epoxy-3,14-dihydroxy-17-methylmorphinan-6-one;Dihydroxymorphinone
- CAS NO.:76-41-5
- Empirical Formula: C17H19NO4
- Molecular Weight: 301.34
- MDL number: MFCD00079194
- EINECS: 200-959-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
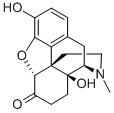
What is OXYMORPHONE?
Toxicity
Oxymorphone overdosage is characterized by respiratory depression, extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. Patients experiencing an overdose may develop apnea, circulatory collapse, and cardiac arrest. Intravenous mouse LD50 is 172 mg/kg.
Description
Oxymorphone is a 60-year-old opioid analgesic. Its structure is that of morphine with the double bond hydrogenated and an additional hydroxyl group that makes it 10 times more potent than morphine.
In 1955, Ulrich Weiss at the New York Botanical Garden (of all places) began a series of articles on new morphine derivatives. In the first article of the series, he described the?synthesis of 14-hydroxydihydromorphinone, which later became known as oxymorphone. Weiss went on to publish five more articles on morphine derivatives.
In 1957, a patent on the synthesis was awarded to Weiss and Mozes Juda Lewenstein. (The patent application preceded Weiss’s article.) The patent was assigned to Lewenstein of Endo Pharmaceuticals (New York). Endo began to market the drug in the United States in 1959.
In June of this year, the US Food and Drug Administration, concerned about the widespread abuse of opioids, requested that the manufacturer (now Endo International, based in Dublin, Ireland)?withdraw oxymorphone-containing Opana ER?from the market. Although Endo formulated the solid pill Opana ER to prevent users from snorting or injecting it, people still figured out ways to abuse the drug.
In July, Endo “voluntarily”?removed Opana ER?while stressing that the drug is safe and efficacious when used properly.
Chemical properties
Crystalline Solid
Originator
Numorphan,Endo, US ,1959
The Uses of OXYMORPHONE
Controlled substance (opiate). Analgesic (narcotic)
Background
An opioid analgesic with actions and uses similar to those of morphine, apart from an absence of cough suppressant activity. It is used in the treatment of moderate to severe pain, including pain in obstetrics. It may also be used as an adjunct to anesthesia (From Martindale, The Extra Pharmacopoeia, 30th ed, p1092). On June 8, 2017, FDA requested Endo Pharmaceuticals to remove the medication from the market due to opioid misuse and abuse risks associated with the product's injectable reformulation.
Indications
For the treatment of moderate-to-severe pain.
Definition
ChEBI: Oxymorphone is a morphinane alkaloid.
Manufacturing Process
Thebaine is dissolved in aqueous formic acid and treated with 30% H2O2; neutralization with aqueous ammonia gives 14-hydroxycodeinone. It is hydrogenated to give oxycodone. 90 ml of concentrated hydrobromic acid are heated to 90°C. 9 grams of 14-hydroxydihydrocodeinone (oxycodone) are then added under stirring and the mixture is quickly heated to 116°C and kept at this temperature under reflux condenser for 20 minutes, with continued stirring. The resulting brown solution is diluted with about 90 ml of water and chilled with ice. Aqueous 10% sodium hydroxide solution is now added to alkaline reaction and the liquid is extracted 3 times with 100 cc portions of chloroform. The layers are separated and the aqueous phase is filtered and acidified by the addition of concentrated aqueous hydrochloric acid, treated with charcoal and filtered.
The filtrate is treated with concentrated aqueous ammonia until the mixture gives a pink color on phenolphthalein paper. The liquid is extracted seven times with 100 cc portions of chloroform, the extracts are combined, dried with anhydrous sodium sulfate and evaporated. The residue is dissolved in ethanol by refluxing and the ethanol evaporated nearly to dryness. 100 cc of benzene are then added, the mixture is refluxed for ? hour and set aside for crystallization. After cooling, the desired compound is collected by filtration, 2.3 grams of a white crystalline powder are obtained; MP 245° to 247°C. This powder consisting of 14-hydroxydihydromorphinone can be purified by recrystallization from benzene, ethylacetate or ethanol. From benzene it generally forms diamond shaped platelets, while needles are obtained from ethylacetate.
On heating, the crystals are discolored from about 200°C on, and melt at 246° to 247°C to a black liquid, which decomposes with strong volume increase if the temperature is raised further by a few degrees.
brand name
Numorphan (Endo); Opana (Endo).
Therapeutic Function
Narcotic analgesic
Biological Functions
Oxymorphone is 10 times as potent as morphine, with actions similar to those of hydromorphone. Oxymorphone, however, has little antitussive activity, and as such is a useful analgesic in patients with pulmonary disease who need to retain the ability to cough.
General Description
Oxymorphone is the 14 beta-hydroxyl version of hydromorphone,analogous to the hydrocodone, oxycodone pair discussedabove. Although the addition of the 14 beta-hydroxylgroup to hydrocodone (30 mg) yielded oxycodone (20 mg),a more potent drug, the opposite is true for the conversion ofhydromorphone (7.5 mg) to oxymorphone (10 mg). The reasonfor this is that the oral bioavailability of oxymorphone(10%) is lower than that of hydromorphone (35%) becauseof decreased absorption and increased first-pass metabolism.Presumably, the addition of the OH group does increaseits binding affinity at the receptor as the injectableform of oxymorphone (1 mg) is more potent than injectablehydromorphone (1.5 mg).
Oxymorphone is available as a suppository (5 mg), an injection(1 mg/mL), an immediate-release tablet (5 mg, 10mg), and in 2003 the FDA approved a sustained release formulation(Opana ER 5 mg, 10 mg, 20 mg, and 40 mg). The12-hour coverage of the extended release tablet provides anotheroption for those patients suffering from chronic pain.The side effect profile of the extended release formulationsof morphine, oxycodone, and oxymorphone are similar, andthere appears to be no clear advantage of one over the other.
Pharmacokinetics
Oxymorphone is a semi-synthetic opioid substitute for morphine. It is a potent analgesic. Opioid analgesics exert their principal pharmacologic effects on the CNS and the gastrointestinal tract. The principal actions of therapeutic value are analgesia and sedation. Opioids produce respiratory depression by direct action on brain stem respiratory centers. The mechanism of respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Metabolism
Oxymorphone undergoes extensive hepatic metabolism in humans. After a 10 mg oral dose, 49% was excreted over a five-day period in the urine. Of this, 82% was excreted in the first 24 hours after administration. The recovered drug-related products contained the oxymorphone (1.9%), the conjugate of oxymorphone (44.1%), the 6(beta)-carbinol produced by 6-keto reduction of oxymorphone (0.3%), and the conjugates of 6(beta)-carbinol (2.6%) and 6(alpha)-carbinol (0.1%).
Properties of OXYMORPHONE
| Melting point: | 248-249°C (dec) |
| Boiling point: | 518.6±50.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | Soluble in DMSO |
| form | Powder |
| appearance | white crystals |
| pka | pKa 8.50 (Uncertain);9.33 (Uncertain) |
| EPA Substance Registry System | Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, (5.alpha.)- (76-41-5) |
Safety information for OXYMORPHONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for OXYMORPHONE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![OXYCODONE HYDROCHLORIDE, [7,8-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB5130533.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1