Oxamic acid
Synonym(s):Aminooxoacetic acid;Oxalic acid monoamide
- CAS NO.:471-47-6
- Empirical Formula: C2H3NO3
- Molecular Weight: 89.05
- MDL number: MFCD00008006
- EINECS: 207-443-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-18 10:52:13
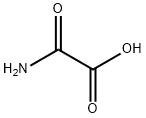
What is Oxamic acid?
Description
Oxamic acids also known to as oxalic acid monoamides have emerged as potent precursors for the generation of the carbamoyl radical. Oxamic acids can easily undergo decarboxylation through a single electron oxidation resulting in the generation of the reactive carbamoyl radical, which can then engage in diverse radical reactions or undergo a second single electron oxidation as originally unveiled by Minisci. Oxamic acids are thus versatile intermediates for the synthesis of nitrogen-containing organic molecules[1]. The oxidative decarboxylation of oxamic acids can be mediated through thermal, photochemical, electrochemical or photoelectrochemical means, generating carbamoyl radicals, which may further add to unsaturated systems to provide a broad range of important amides. Oxidative decarboxylation of oxamic acids also offers a straightforward entry for the preparation of urethanes, ureas, and thioureas.
Chemical properties
Oxamic acid is a white, water-soluble solid. It is the monoamide ofoxalic acid. It can react with metal carbonates to form oxamate. Oxamic acid inhibits lactate dehydrogenase A.
The Uses of Oxamic acid
Oxamic acid is an ozone oxidation product and is used in the synthesis of hydroxybenzimidazoles preparation as potential anti tumor agents targeting human lactate dehydrogenase A.This compound is suitable for lactate dehydrogenase (LDH) related research.
What are the applications of Application
Oxamic acid is an amino-substituted glyoxylic acid derivative
Definition
ChEBI: Oxamic acid is a dicarboxylic acid monoamide resulting from the formal condensation of one of the carboxy groups of oxalic acid with ammonia. It has a role as an Escherichia coli metabolite. It is a conjugate acid of an oxamate.
What are the applications of Application
Oxamic acid (OA) has applications in polymer chemistry. It increases the water solubility of certain polymers, including polyester,epoxide, and acrylic upon binding with them. Oxamic acid can be used as a reactant to prepare 6-phenanthridinecarboxamide by direct C-H carbamoylation reaction using ammonium persulfate in DMSO. It can also be used as an organic ligand to prepare functionalized metal oxide nanoparticles for various biological applications. OA along with p-aminobenzoic acid is used to functionalize Au nanoparticles for the development of a sensor to detect Fe3+ ions by the calorimetric method.
References
[1] Ikechukwu Martin Ogbu . “Oxamic acids: useful precursors of carbamoyl radicals.” Chemical Communications 58 55 (2022): Pages 7593-7607.
Properties of Oxamic acid
| Melting point: | 207-210 °C (dec.) (lit.) |
| Boiling point: | 165.08°C (rough estimate) |
| Density | 1.6193 (rough estimate) |
| refractive index | 1.4264 (estimate) |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Crystalline Powder |
| pka | 1.60±0.20(Predicted) |
| color | White |
| Water Solubility | Soluble in water 108 mg/mL. |
| Merck | 14,6917 |
| BRN | 1743294 |
| InChI | InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) |
| CAS DataBase Reference | 471-47-6(CAS DataBase Reference) |
| EPA Substance Registry System | Oxamic acid (471-47-6) |
Safety information for Oxamic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Oxamic acid
| InChIKey | SOWBFZRMHSNYGE-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C(N)=O |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![ETHYL 2-([1-(4-CHLOROPHENYL)-1-METHYL-1-OXO-LAMBDA6-SULFANYLIDENE]AMINO)-2-OXOACETATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9427882.gif)



You may like
-
 Oxamic Acid CAS 471-47-6View Details
Oxamic Acid CAS 471-47-6View Details
471-47-6 -
 Oxamic acid 95% CAS 471-47-6View Details
Oxamic acid 95% CAS 471-47-6View Details
471-47-6 -
 Oxamic acid CAS 471-47-6View Details
Oxamic acid CAS 471-47-6View Details
471-47-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4