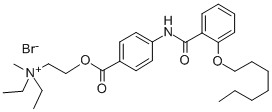
Otilonium bromide
Synonym(s):N,N-Diethyl-N-methyl-2-[(4-{[2-(octyloxy)benzoyl]amino}benzoyl)oxy]ethanaminium bromide;N,N-Diethyl-N-methyl-2-[[4-[[2-(octyloxy)benzoyl]amino]benzoyl]oxy]-ethanaminium bromide;Octylonium bromide
- CAS NO.:26095-59-0
- Empirical Formula: C29H43BrN2O4
- Molecular Weight: 563.58
- MDL number: MFCD00211142
- EINECS: 247-457-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-03 18:47:13

What is Otilonium bromide?
Description
Otilonium is an inhibitor of L-type (IC50 = 2.3 μM) and T-type calcium channels (IC50s = 0.8, 1.1, and 0.4 μM for Cav3.1, Cav3.2, and Cav3.3, respectively). It is also an antagonist of muscarinic acetylcholine receptors (mAChRs; IC50s = 0.054, 0.4, 0.222, and 0.156 μM for M1, M2, M4, and M5 muscarinic receptors, respectively) and the platelet activating factor (PAF) receptor (IC50 = 0.0552 μM). Otilonium inhibits the inward calcium current in isolated rat colonic smooth muscle cells (EC50 = 885 nM), an effect that can be blocked by the L-type calcium channel inhibitor nifedipine . It inhibits contractions induced by the muscarinic acetylcholine receptor (mAChR) agonist methacholine in isolated circular muscle of the guinea pig proximal colon (IC50 = 3.7 μM). Otilonium (4 mg/kg) decreases fecal excretion and wet dog shakes and increases the tail withdrawal latency in the tail-flick test in a rat model of opioid withdrawal syndrome induced by morphine and naloxone.
Chemical properties
White Powder
Originator
Spasmomen,Minapharm Co.
The Uses of Otilonium bromide
Otilonium bromide is an antimuscarinic
The Uses of Otilonium bromide
Inhibits platlet activating factor induced hypotension. Antispasmodic
The Uses of Otilonium bromide
Octylonium bromide is an antimuscarinic. Octylonium bromide is a platelet-activating factor antagonist as analgesic, anti-inflammatory, uterine contraction inhibiting, and anti-tumor agent. m bromide is used as an antispasmodic.
The Uses of Otilonium bromide
antimuscarinic used as a spasmolytic agent
What are the applications of Application
Octylonium Bromide is a PAF-R inhibitor
Manufacturing Process
3 Methods of producing of p-[2-(n-octyloxy)benzoyl]aminobenzoate of Ndiethylammoniumethanol:
1. 21.20 g (0.1 mole) of o-octyloxybenzoyl chloride and aqueous 10% NaOH are added at room temperature, with stirring and by slow dropping to 23.63 g (0.1 mole) of 2-diethylamine-ethyl-p-aminobenzoate in 100 ml of water, in such a manner as to keep the reaction mixture slightly alkaline. After concluding the slow dropping the solution is kept under stirring for 1 h and then the precipitate is collected. This precipitate, p-[2-(n-octyloxy)benzoyl] aminobenzoate of N-diethylammoniumethanol dried and recrystallized from hexane, has a melting point of 81°-82°C.
2. To 31.3 g (0.1 mole) of p-[2-(n-octyloxy)benzoyl]aminobenzoate acid in 300 ml of ethanol, are added 4.0 g (0.1 mole) of finely ground NaOH and the whole is heated to reflux for 1 h. Then 20.25 g (0.15 mol) of 2
Trade diethylaminoethyl chloride are slowly dropped under stirring and the heating is continued for 4 h. After cooling, the sodium chloride formed is filtered off and the solvent is separated by distillation, and the excess of the base, under a reduced pressure. The residue of p-[2-(n-tyloxy)benzoyl]aminobenzoate of Ndiethylammoniumethanol, recrystallized from hexane, has a melting point of 81°-82°C.
3. 11.7 g (0.1 mole) of N-diethylaminoethanol in 200 ml of anhydrous pyridine are added by careful dropping, 34.7 g (0.1 mole) of the chloride of p[2-(n-octyloxy)benzoyl]aminobenzoate acid and the mixture is heated in a water-bath for 3 h. The solvent is then separated by vacuum concentration, the residue is taken up with water, alkalinized and extracted with ether. The collected ether extracts, anhydridised owing to the separation of the solvent, leave a residue of p-[2-(n-tyloxy)benzoyl]aminobenzoate of Ndiethylammoniumethanol which, recrystallized from hexane, has a melting point of 81°-82°C.
p-[2-(n-Octyloxy)benzoyl]aminobenzoate of N-diethylmethylammoniumethyl bromide may be prepared by reaction of the p-[2-(n-octyloxy) benzoylaminobenzoate of N-diethylammoniumethanol with methylating agents such as methylbromide
Therapeutic Function
Anticholinergic, Spasmolytic
General Description
Otilonium bromide is an antispasmodic. It contains a red color ACh (acetylcholine) like moiety bound to an aromatic system that ends with an octyl ether.
Biochem/physiol Actions
Otilonium Bromide is a muscarinic receptor antagonist (antimuscarinic). Otilonium Bromide is a quaternary ammonium salt that exerts spasmolytic action selective on the distal gastrointestinal tract. It is used world-wide for the treatment of irritable bowel syndrome (IBS).
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. When heated to decomposition it emits toxic fumes of Brí and NOx.
Properties of Otilonium bromide
| Melting point: | 130-133°C |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 26095-59-0(CAS DataBase Reference) |
Safety information for Otilonium bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Otilonium bromide
Otilonium bromide manufacturer
Zydus Lifesciences Ltd.
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran

![N-[(2-methoxyphenyl)methyl]-4-methylaniline](https://img.chemicalbook.in/CAS/GIF/130191-24-1.gif)
You may like
-
 26095-59-0 Otilonium bromide 98%View Details
26095-59-0 Otilonium bromide 98%View Details
26095-59-0 -
 26095-59-0 98%View Details
26095-59-0 98%View Details
26095-59-0 -
 Otilonium bromide 98% CAS 26095-59-0View Details
Otilonium bromide 98% CAS 26095-59-0View Details
26095-59-0 -
 Otilonium bromide 98% (HPLC) CAS 26095-59-0View Details
Otilonium bromide 98% (HPLC) CAS 26095-59-0View Details
26095-59-0 -
 Otilonium bromide CAS 26095-59-0View Details
Otilonium bromide CAS 26095-59-0View Details
26095-59-0 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0