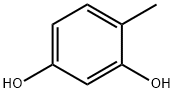
Orcinol
Synonym(s):3,5-Dihydroxytoluene;5-Methylresorcinol;5-Methylresorcinol, Orcinol
- CAS NO.:504-15-4
- Empirical Formula: C7H8O2
- Molecular Weight: 124.14
- MDL number: MFCD00002291
- EINECS: 207-984-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
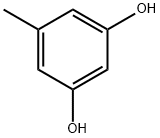
What is Orcinol?
Chemical properties
pink-grey to pink-brown powder or crystals.
The Uses of Orcinol
Orcinol can be used to synthesize:
Orcinol-containing azacryptands for use in optical amplifiers and light-emitting devices.
Ternary co-crystal with 4,4′-bipyridine.
Low-density carbon aerogels in the presence of formaldehyde.
PEG-orcinol coumarins with potent tyrosinase inhibitory activity.
What are the applications of Application
Orcinol is a useful phenol for proteomics research
Definition
ChEBI: Orcinol is a 5-alkylresorcinol in which the alkyl group is specified as methyl. It has a role as an Aspergillus metabolite. It is a 5-alkylresorcinol and a dihydroxytoluene.
What are the applications of Application
Orcinol is found in many lichen species and It is used as an analytical reagent for pentoses, lignin, beet sugar, saccharoses, arabinose, and diastase. The orcinol assay was used for the direct assay of the number of glycolipids presents in the sample. The orcinol reagent was prepared by adding concentrated sulphuric acid, H2SO4 (98% w/w) and 0.19% orcinol (3,5-dihydroxytoluene) to distilled water.
Preparation
Orcinol has been isolated from numerous lichen fungi (Robiquet, 1829) and can be synthesized by decarboxylation of orsellinic acid in Umbilicaria papulosa and Gliocladium roseum (Pettersson, 1965; Mosbach and Ehrensvard, 1966).
Orcinol was first prepared by dehydroacetic acid, a conversion that involved ring-opening of the pyrone to a triketone. This early experiment helped establish the rich condensation chemistry of polyketides. It can be obtained by fusing extract of aloes with potash, followed by acidification.
US3865884A: Preparation of orcinol
Biological Activity
Orcinol is a compound obtained from DHA which can mimic the biogenetic synthesis of phenolic compounds.
Orcinol is a polyketide synthase-derived phenol that has been found in F. graminearum and has diverse biological activities. It scavenges DPPH radicals (IC50 = 2.93 mM). Orcinol (2.5 and 5 mg/kg) increases the number of entries into and percentage of time spent in the open arms of the elevated plus maze in mice, indicating anxiolytic-like activity. It has also been used in the colorimetric detection of carbohydrates.
Safety Profile
Poison by subcutaneous and intravenous routes. Moderately toxic by ingestion and intraperitoneal routes. Mildly toxic by skin contact. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise orcinol from CHCl3/*benzene (2:3). See hydrate in previous entry. [Beilstein 6 H 882, 6 IV 5892.]
Properties of Orcinol
| Melting point: | 106-112 °C(lit.) |
| Boiling point: | 290 °C |
| Density | 1.2900 |
| refractive index | 1.4922 (estimate) |
| Flash point: | 159 °C |
| storage temp. | Store at <= 20°C. |
| solubility | 80g/l |
| form | Crystalline Powder or Crystals |
| pka | 9.56±0.10(Predicted) |
| color | Pink-gray to pink-brown |
| Sensitive | Air Sensitive |
| Merck | 14,6864 |
| BRN | 1071903 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 504-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 3,5-Dihydroxytoluene(504-15-4) |
| EPA Substance Registry System | 1,3-Benzenediol, 5-methyl- (504-15-4) |
Safety information for Orcinol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Orcinol
| InChIKey | OIPPWFOQEKKFEE-UHFFFAOYSA-N |
Orcinol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Orcinol Anhydrous extrapure (3,5- dihydroxytoluene) CAS 504-15-4View Details
Orcinol Anhydrous extrapure (3,5- dihydroxytoluene) CAS 504-15-4View Details
504-15-4 -
 Orcinol, anhydrous CAS 504-15-4View Details
Orcinol, anhydrous CAS 504-15-4View Details
504-15-4 -
 3,5-Dihydroxytoluene CAS 504-15-4View Details
3,5-Dihydroxytoluene CAS 504-15-4View Details
504-15-4 -
 5-Methylresorcinol Anhydrous CAS 504-15-4View Details
5-Methylresorcinol Anhydrous CAS 504-15-4View Details
504-15-4 -
 Orcinol Anhydrous (3,5- dihydroxytoluene) CAS 504-15-4View Details
Orcinol Anhydrous (3,5- dihydroxytoluene) CAS 504-15-4View Details
504-15-4 -
 Orcinol CASView Details
Orcinol CASView Details -
 3,5-Dihydroxytoluene CAS 504-15-4View Details
3,5-Dihydroxytoluene CAS 504-15-4View Details
504-15-4 -
 OrcinolView Details
OrcinolView Details
504-15-4
