Omarigliptin (MK-3102)
- CAS NO.:1226781-44-7
- Empirical Formula: C17H20F2N4O3S
- Molecular Weight: 398.43
- MDL number: MFCD22573261
- EINECS: 682-558-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
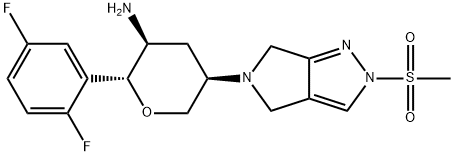
What is Omarigliptin (MK-3102)?
Description
Merck earned its first global approval for omarigliptin in Japan in 2015, and phase III development is ongoing in other countries around the globe for this interesting small molecule DPP-4 inhibitor. Interestingly, while most DPP-4 inhibitors used to treat type 2 DM require daily administration, omarigliptin is a weekly treatment. The process-scale synthesis of omarigliptin has been nicely described in an October 2015 paper from the Merck process group.
Description
MK-3102 is a potent, reversible, and competitive inhibitor of dipeptidyl peptidase 4 (DPP-4; IC50 = 1.6 nM; Ki = 0.8 nM). It is selective for DPP-4 over 168 proteases, ion channels, and enzymes with IC50 values greater than 10 μM in all assays. MK-3102 significantly reduces blood glucose levels in a dose-dependent manner in vivo in rats. It also has a long half-life (11 and 22 hours in rat and dog, respectively) making it suitable for once weekly dosing. Clinical trials demonstrate that formulations containing MK-3102 reduce plasma glucose and HbA1c in patients with type 2 diabetes mellitus (T2DM).
The Uses of Omarigliptin (MK-3102)
Omarigliptin is a potent and selective dipeptidyl peptidase 4 (DPP-4) inhibitor to be used as treatment for type 2 diabetes.
Definition
ChEBI: Omarigliptin is a pyrrolopyrazole.
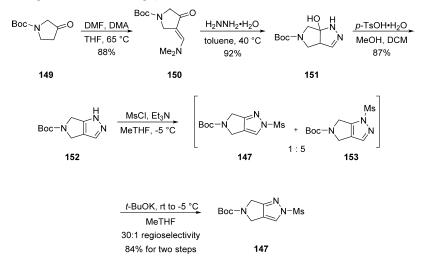
Synthesis
The synthesis began with the efficient condensation of
pyrrolidinone 149 with dimethylformamide-dimethylacetal
(DMF-DMA) to afford enaminoketone 150 in 88% yield. Subsequent condensation with hydrazine
monohydrate gave tertiary alcohol 151 in 92% yield, and this
step was followed by acid-promoted dehydration to afford fused
pyrazole 152. An initial kinetic mesylation delivered a 1:5 ratio
of 147:153, in favor of the undesired regioisomer. However,
when the crude mixture was warmed to ambient temperature
and treated with potassium tert-butoxide, thermodynamic
equilibration provided the more stable N1-mesylate 147. This
process furnished the desired regioisomer 147 in a 30:1 ratio
and 84% yield over the two steps. Reaction
monitoring by HPLC suggests that cleavage of the mesyl group
of 153 results in anion formation on the adjacent nitrogen,
which then allows for mesylation at the desired position.
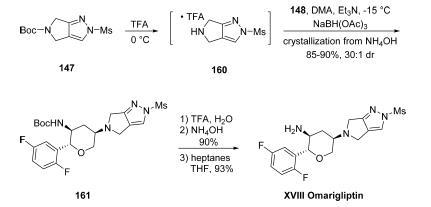
Ester 154 was subjected to a
three-step sequence whereby alkylation with propargyl besylate
followed by saponification with sodium hydroxide and Boc
protection resulted in amide 155 in 75% yield over three steps.
The Weinreb amide was then subjected to the Knochel ?°turbo
Grignard?± reagent derived from 1-bromo-2,4-difluorobenzene
to provide ketone 156 in 89% yield. An enantioselective
transfer hydrogenation was carried out utilizing (R,R)-Ts-
DENEB as the chiral induction reagent to afford intermediate
157 in excellent yield and enantio- and diastereoselectivity,
which underwent ruthenium-mediated cyclization with the
pendant alkyne to afford dihydropyran 158 in 86% yield. A
two-step hydroboration/oxidation involving the endocyclic
vinyl ether furnished 159 as a mixture of diastereomers in
89% yield, and this was followed by RuCl3/NaBO3-mediated
oxidation to provide the lactone fragment 148 in 80% yield.

Removal of the Boc group within 147 was
effected upon treatment with TFA, affording intermediate 160,
which was not isolated but instead exposed to ketone 148
under reductive amination conditions to afford diaminopyran 161 in excellent yield and diastereoselectivity (30:1 dr). Finally,
Boc deprotection and crystallization from THF/heptanes
furnished omarigliptin in an impressive 45% yield over its
nine-step longest linear sequence.
in vitro
mk-3102 is a competitive, reversible inhibitor of dpp-4 and is more potent than sitagliptin. it is highly selective over all proteases tested, including qpp, fap, pep, dpp8, and dpp9. the compound has weak ion channel activity [1].
in vivo
mk-3102 was evaluated for its ability to improve glucose tolerance in lean mice. when orally administered 1 h prior to dextrose challenge in an oral glucose tolerance test, it significantly reduced blood glucose excursion in a dosedependent manner from 0.01 mg/kg to 0.3 mg/kg [1].
References
[1] biftu t, sinha-roy r, chen p, qian x, feng d, kuethe jt, scapin g, gao yd, yan y, krueger d, bak a, eiermann g, he j, cox j, hicks j, lyons k, he h, salituro g, tong s, patel s, doss g, petrov a, wu j, xu ss, sewall c, zhang x, zhang b, thornberry na, weber ae. omarigliptin (mk-3102): a novel long-acting dpp-4 inhibitor for once-weekly treatment of type 2 diabetes. j med chem. 2014 apr 24;57(8):3205-12.
Properties of Omarigliptin (MK-3102)
| Boiling point: | 529.4±60.0 °C(Predicted) |
| Density | 1.61±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | insoluble in EtOH; insoluble in H2O; ≥17.15 mg/mL in DMSO |
| form | solid |
| pka | 9.11±0.60(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 |
| CAS DataBase Reference | 1226781-44-7 |
Safety information for Omarigliptin (MK-3102)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Omarigliptin (MK-3102)
| InChIKey | MKMPWKUAHLTIBJ-ISTRZQFTSA-N |
| SMILES | [C@H]1(C2=CC(F)=CC=C2F)OC[C@H](N2CC3=CN(S(C)(=O)=O)N=C3C2)C[C@@H]1N |
Omarigliptin (MK-3102) manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Omarigliptin 95% CAS 1226781-44-7View Details
Omarigliptin 95% CAS 1226781-44-7View Details
1226781-44-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
