Odevixibat
Synonym(s):(2S)-2-[(2R)-2-(2-{[3,3-Dibutyl-7-(methylsulfanyl)-1,1-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-1λ6,2,5-benzothiadiazepin-8-yl]oxy}acetamido)-2-(4-hydroxyphenyl)acetamido]butanoic acid;(2S)-2-[[(2R)-2-[[2-[[3,3-Dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl]oxy]acetyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]butanoic acid;
- CAS NO.:501692-44-0
- Empirical Formula: C37H48N4O8S2
- Molecular Weight: 740.93
- MDL number: MFCD32710220
- Update Date: 2024-07-02 08:55:16
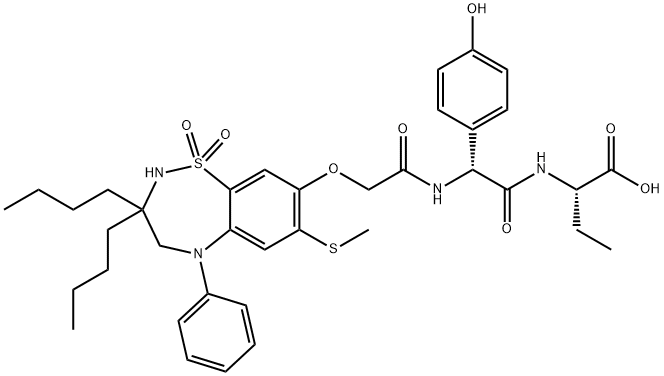
What is Odevixibat?
Absorption
A 7.2 mg single oral dose of odevixibat in adults reaches a Cmax of 0.47 ng/mL, with an AUC0-24h of 2.19 h*ng/mL. The majority of adult and pediatric patients, given a therapeutic dose, do not have detectable plasma concentrations of odevixibat.
Toxicity
Data regarding overdoses of odevixibat are not readily available, due to the low systemic absorption of the drug. If patients experience an overdose, initiate treatment with symptomatic and supportive measures.
Indications
Odevixibat is indicated for the treatment of pruritus in patients older than 3 months with progressive familiar intrahepatic cholestasis (PFIC) and cholestatic pruritus in patients 12 months of age and older with Alagille Syndrome. It may not be effective in patients with PFIC type 2 with ABCB11 variants since these patients lack a functional bile salt export pump.
Background
Odevixibat, or A4250, is an ileal sodium/bile acid cotransporter inhibitor indicated for the treatment of pruritus in patients older than 3 months, with progressive familiar intrahepatic cholestasis (PFIC). Odevixibat is the first approved non-surgical treatment option for PFIC. Previous therapies for PFIC included a bile acid sequestrant such as ursodeoxycholic acid.
Odevixibat was granted FDA approval on 20 July 2021.
Pharmacokinetics
Odevixibat, or A4250, is an ileal sodium/bile acid cotransporter inhibitor indicated for the treatment of pruritus in patients older than 3 months, with progressive familiar intrahepatic cholestasis (PFIC). It has a moderate duration of action as it is given once daily. Odevixibat has a wide therapeutic index as patients were given single doses up to 10 mg while the maximum therapeutic dose is 6 mg daily. Patients should be counselled regarding the risks of elevated liver function tests, diarrhea, and fat soluble vitamin defiencies.
Metabolism
Odevixibat is largely unmetabolized, however a small amount is metabolized in vitro by mono-hydroxylation. The exact structure of the metabolite has not been characterized as a primary endpoint of the clinical trial was to characterize the structure of metabolites accounting for >10% of the dose in plasma, urine, or feces. No metabolites have been identified at such a high concentration.
Properties of Odevixibat
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C, stored under nitrogen |
| solubility | DMSO : 166.67 mg/mL (224.95 mM; Need ultrasonic) |
| pka | 3.32±0.10(Predicted) |
| form | A solid |
| color | White to off-white |
Safety information for Odevixibat
Computed Descriptors for Odevixibat
Odevixibat manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateYou may like
-
 501692-44-0 Odevixibat 98%View Details
501692-44-0 Odevixibat 98%View Details
501692-44-0 -
 Odevixibat CAS 501692-44-0View Details
Odevixibat CAS 501692-44-0View Details
501692-44-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8