501692-44-0
Product Name:
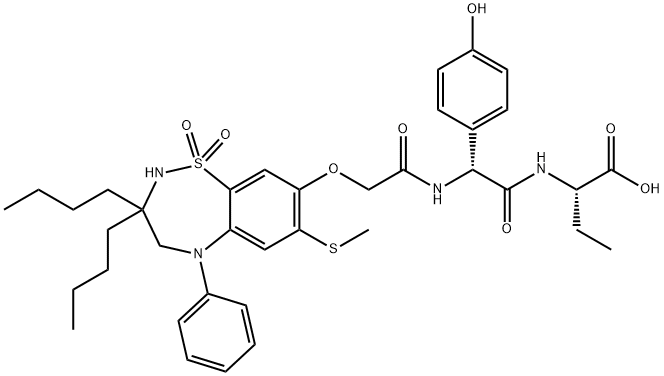
Odevixibat
Formula:
C37H48N4O8S2
Synonyms:
(2S)-2-[(2R)-2-(2-{[3,3-Dibutyl-7-(methylsulfanyl)-1,1-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-1λ6,2,5-benzothiadiazepin-8-yl]oxy}acetamido)-2-(4-hydroxyphenyl)acetamido]butanoic acid;(2S)-2-[[(2R)-2-[[2-[[3,3-Dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl]oxy]acetyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]butanoic acid;
Inquiry
COMPUTED DESCRIPTORS
| Molecular Weight | 740.9 g/mol |
|---|---|
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 17 |
| Exact Mass | 740.29135685 g/mol |
| Monoisotopic Mass | 740.29135685 g/mol |
| Topological Polar Surface Area | 208 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Odevixibat, or A4250, is an ileal sodium/bile acid cotransporter inhibitor indicated for the treatment of pruritus in patients older than 3 months, with progressive familiar intrahepatic cholestasis (PFIC). Odevixibat is the first approved non-surgical treatment option for PFIC. Previous therapies for PFIC included a bile acid sequestrant such as [ursodeoxycholic acid]. Odevixibat was granted FDA approval on 20 July 2021.