o-Xylene
Synonym(s):o-Xylene;1,2-Dimethylbenzene;Dimethylbenzenes, Dimethylbenzene;Xylene mixture of isomers
- CAS NO.:95-47-6
- Empirical Formula: C8H10
- Molecular Weight: 106.17
- MDL number: MFCD00008519
- EINECS: 202-422-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
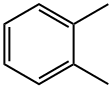
What is o-Xylene?
Chemical properties
colourless liquid
Physical properties
Clear, colorless liquid with an aromatic odor. An odor threshold concentration of 380 ppbv was reported by Nagata and Takeuchi (1990).
The Uses of o-Xylene
o-Xylene is largely used in the production of phthalic anhydride, and is generally extracted by distillation from a mixed Xylene stream in a plant primarily designed for p-Xylene production.
The Uses of o-Xylene
Preparation of phthalic acid, phthalic anhydride, terephthalic acid, isophthalic acid; solvent for alkyd resins, lacquers, enamels, rubber cements; manufacture of dyes, pharmaceuticals, and insecticides; motor fuels.
The Uses of o-Xylene
Xylene, xylenes, and total xylenes are used interchangeably since xylene usually exists as a mixture of three isomers: 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene, i.e., o-, m-, and p-xylene, and is usually used as a mixture of the three forms. The mixture often also contains ethylbenzene. It is a high volume industrial chemical used in the synthetic fiber, chemical and plastics industries and as a solvent, cleaning agent and thinner for paints and varnishes.
What are the applications of Application
o-Xylene is a major petrochemicals, produced by catalytic reforming
Definition
ChEBI: A xylene substituted by methyl groups at positions 1 and 3.
Synthesis Reference(s)
Journal of the American Chemical Society, 97, p. 7262, 1975 DOI: 10.1021/ja00858a011
The Journal of Organic Chemistry, 44, p. 2185, 1979 DOI: 10.1021/jo01327a032
General Description
A colorless watery liquid with a sweet odor. Less dense than water. Insoluble in water. Irritating vapor.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1,2-Dimethylbenzene may react with oxidizing materials. .
Health Hazard
Vapors cause headache and dizziness. Liquid irritates eyes and skin. If taken into lungs, causes severe coughing, distress, and rapidly developing pulmonary edema. If ingested, causes nausea, vomiting, cramps, headache, and coma. Can be fatal. Kidney and liver damage can occur.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic bj7 intraperitoneal route. Mldly toxic by ingestion and inhalation. An experimental teratogen. A common air contaminant. A very dangerous fire hazard when exposed to heat or flame. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use foam, CO2, dry chemical. Incompatible with oxidzing materials. When heated to decomposition it emits acrid smoke and irritating fumes. Emitted from modern building materials (CENEAR 69,22,91). See also other xylene entries.
Source
Detected in distilled water-soluble fractions of 87 octane gasoline (3.83 mg/L), 94 octane
gasoline (11.4 mg/L), Gasohol (8.49 mg/L), No. 2 fuel oil (1.73 mg/L), jet fuel A (0.87 mg/L),
diesel fuel (1.75 mg/L), military jet fuel JP-4 (1.99 mg/L) (Potter, 1996), new motor oil (16.2 to 17.5 μg/L), and used motor oil (294 to 308 μg/L) (Chen et al., 1994). The average volume percent
and estimated mole fraction in American Petroleum Institute PS-6 gasoline are 2.088 and 0.01959,
respectively (Poulsen et al., 1992). Schauer et al. (1999) reported o-xylene in a diesel-powered
medium-duty truck exhaust at an emission rate of 830 μg/km. Diesel fuel obtained from a service
station in Schlieren, Switzerland contained o-xylene at a concentration of 223 mg/L (Schluep et
al., 2001).
California Phase II reformulated gasoline contained o-xylene at a concentration of 19.7 g/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 5.41 and 562 mg/km, respectively (Schauer et al., 2002).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average m+p-xylene concentrations reported in water-soluble fractions of unleaded gasoline,
kerosene, and diesel fuel were 8.611, 0.658, and 0.228 mg/L, respectively. When the authors
analyzed the aqueous-phase via U.S. EPA approved test method 610, average m+p-xylene
concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were
lower, i.e., 6.068, 0.360, and 0.222 mg/L, respectively.
Based on laboratory analysis of 7 coal tar samples, o-xylene concentrations ranged from 2 to
2,000 ppm (EPRI, 1990). A high-temperature coal tar contained o-xylene at an average
concentration of 0.04 wt % (McNeil, 1983).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of o-xylene was 18.1 mg/kg of pine burned. Emission rates of o-xylene were not measured
during the combustion of oak and eucalyptus.
Drinking water standard (final): For all xylenes, the MCLG and MCL are both 10 mg/L. In
addition, a DWEL of 70 mg/L was recommended (U.S. EPA, 2000).
Environmental Fate
Biological. Reported biodegradation products of the commercial product containing xylene
include α-hydroxy-p-toluic acid, p-methylbenzyl alcohol, benzyl alcohol, 4-methylcatechol, mand
p-toluic acids (Fishbein, 1985). o-Xylene was also cometabolized resulting in the formation of
o-toluic acid (Pitter and Chudoba, 1990). In anoxic groundwater near Bemidji, MI, o-xylene
anaerobically biodegraded to the intermediate o-toluic acid (Cozzarelli et al., 1990). In gasolinecontaminated
groundwater, methylbenzylsuccinic acid was identified as the first intermediate
during the anaerobic degradation of xylenes (Reusser and Field, 2002).
Photolytic. Cox et al. (1980) reported a rate constant of 1.33 x 10-11 cm3/molecule?sec for the
reaction of gaseous o-xylene with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for
the reaction of ethylene with OH radicals.
Surface Water. The evaporation half-life of o-xylene in surface water (1 m depth) at 25 °C is
estimated to be 5.18 h (Mackay and Leinonen, 1975).
Groundwater. Nielsen et al. (1996) studied the degradation of o-xylene in a shallow,
glaciofluvial, unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm study, a cylinder that was open at the bottom and screened at the top was installed through a cased
borehole approximately 5 m below grade. Five liters of water was aerated with atmospheric air to
ensure aerobic conditions were maintained. Groundwater was analyzed weekly for approximately
3 months to determine o-xylene concentrations with time. The experimentally determined firstorder
biodegradation rate constant and corresponding half-life following a 7-d lag phase were
0.1/d and 6.93 d, respectively.
Photolytic. When synthetic air containing gaseous nitrous acid and o-xylene was exposed to
artificial sunlight (λ = 300–450 nm) biacetyl, peroxyacetal nitrate, and methyl nitrate formed as
products (Cox et al., 1980). A n-hexane solution containing o-xylene and spread as a thin film (4
mm) on cold water (10 °C) was irradiated by a mercury medium pressure lamp. In 3 h, 13.6% of
the o-xylene photooxidized into o-methylbenzaldehyde, o-benzyl alcohol, o-benzoic acid, and omethylacetophenone
(Moza and Feicht, 1989). Irradiation of o-xylene at ≈ 2537 ? at 35 °C and 6
mmHg isomerizes to m-xylene (Calvert and Pitts, 1966). Glyoxal, methylglyoxal, and biacetyl
were produced from the photooxidation of o-xylene by OH radicals in air at 25 °C (Tuazon et al.,
1986a).
Chemical/Physical. Under atmospheric conditions, the gas-phase reaction of o-xylene with OH
radicals and nitrogen oxides resulted in the formation of o-tolualdehyde, o-methylbenzyl nitrate,
nitro-o-xylenes, 2,3-and 3,4-dimethylphenol (Atkinson, 1990). Kanno et al. (1982) studied the
aqueous reaction of o-xylene and other aromatic hydrocarbons (benzene, toluene, m- and p-xylene,
and naphthalene) with hypochlorous acid in the presence of ammonium ion. They reported that the
aromatic ring was not chlorinated as expected but was cleaved by chloramine forming cyanogen
chloride. The amount of cyanogen chloride formed increased at lower pHs (Kanno et al., 1982). In
the gas phase, o-xylene reacted with nitrate radicals in purified air forming the following products:
5-nitro-2-methyltoluene and 6-nitro-2-methyltoluene, o-methylbenzaldehyde, and an aryl nitrate
(Chiodini et al., 1993).
Purification Methods
o-Xylene (4.4Kg) is sulfonated by stirring for 4hours with 2.5L of conc H2SO4 at 95o. After cooling, and separating the unsulfonated material, the product is diluted with 3L of water and neutralised with 40% NaOH. On cooling, sodium o-xylene sulfonate separates and is recrystallised from half its weight of water. [A further crop of crystals is obtained by concentrating the mother liquor to one-third of its volume.] The salt is dissolved in the minimum amount of cold water, then mixed with the same amount of cold water, and with the same volume of conc H2SO4 and heated to 110o. o-Xylene is regenerated and steam distils. The distillate is saturated with NaCl, the organic layer is separated, dried and redistilled. [Beilstein 5 H 362, 5 I 179, 5 II 281, 5 III 807, 5 IV 917.]
Properties of o-Xylene
| Melting point: | -26--23 °C (lit.) |
| Boiling point: | 143-145 °C (lit.) |
| Density | 0.879 g/mL at 20 °C (lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | <0.1 atm ( 21.1 °C) |
| refractive index | n |
| Flash point: | 90 °F |
| storage temp. | Store below +30°C. |
| solubility | water: partially soluble0.1705 g/L at 25°C |
| form | Solid or Crystalline Powder |
| pka | >15 (Christensen et al., 1975) |
| color | Yellow to beige |
| Odor | Benzene-like; characteristic aromatic. |
| explosive limit | 1.0-7.6%(V) |
| Odor Threshold | 0.38ppm |
| Water Solubility | Sparingly soluble in water. (0.2g/L) |
| λmax | λ: 288 nm Amax: 1.00 λ: 300 nm Amax: 0.40 λ: 325 nm Amax: 0.05 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,10081 |
| BRN | 1815558 |
| Henry's Law Constant | 14.0 at 45.00 °C, 16.4 at 50.00 °C, 19.0 at 55.00 °C, 21.3 at 60.00 °C, 26.3 at 70.00 °C (static headspace-GC, Park et al., 2004) |
| Exposure limits | NIOSH REL: 100 ppm (435 mg/m3), STEL 150 ppm (655 mg/m3), IDLH 900
ppm; OSHA PEL: TWA 100 ppm; ACGIH TLV: TWA 100 ppm, STEL 150 ppm (adopted). |
| Dielectric constant | 2.5699999999999998 |
| Stability: | Stable. Incompatible with oxidizing agents. Flammable. Hygroscopic. |
| CAS DataBase Reference | 95-47-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2-dimethyl-(95-47-6) |
| EPA Substance Registry System | o-Xylene (95-47-6) |
Safety information for o-Xylene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for o-Xylene
| InChIKey | CTQNGGLPUBDAKN-UHFFFAOYSA-N |
o-Xylene manufacturer
ARRAKIS INDUSTRIES LLP
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
![5-[1-(2,3-DiMethylphenyl)ethenyl]iMidazole](https://img.chemicalbook.in/CAS/GIF/1021949-47-2.gif)

You may like
-
 Ortho Xylene 99%View Details
Ortho Xylene 99%View Details -
 Ortho Xylene 99%View Details
Ortho Xylene 99%View Details -
 o-Xylene CAS 95-47-6View Details
o-Xylene CAS 95-47-6View Details
95-47-6 -
 o-Xylene CAS 95-47-6View Details
o-Xylene CAS 95-47-6View Details
95-47-6 -
 Ortho Xylene CASView Details
Ortho Xylene CASView Details -
 Ortho Xylene 99%View Details
Ortho Xylene 99%View Details -
 Xylenes CASView Details
Xylenes CASView Details -
 Ortho Xylene CASView Details
Ortho Xylene CASView Details
