Norgestimate
Synonym(s):(17α)-17-(Acetyloxy)-13-ethyl-18,19-dinorpregn-4-en-20-yn-3-one 3-oxime;Dexnorgestrel acetime;Norgestimate
- CAS NO.:35189-28-7
- Empirical Formula: C23H31NO3
- Molecular Weight: 369.5
- MDL number: MFCD00867874
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
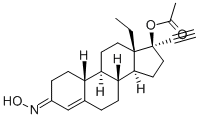
What is Norgestimate?
Absorption
Oral norgestimate has a Tmax of 0.5-2h.
On day 21 of cycle 3, 17-desacetylnorgestimate reaches a Cmax of 1.82ng/mL, with a Tmax of 1.5h, and an AUC of 16.1h*ng/mL. At the same time, norgestrel reaches a Cmax of 2.79ng/mL, with a Tmax of 1.7h, and an AUC of 49.9h*ng/mL.
Toxicity
Data regarding overdoses of norgestimate are rare. However, the majority of patients overdosing on oral contraceptives do not become seriously ill. Treat overdoses with symptomatic and supportive care.
Description
Norgestimate is an orally-effective progestogen, recently launched in combination with ethinyl estradiol as an oral contraceptive.
Chemical properties
White Crystalline Solid
Originator
Ortho (USA)
The Uses of Norgestimate
Progestogen. In combination with estrogen as oral contraceptive
The Uses of Norgestimate
antifungal
The Uses of Norgestimate
Progestogen. In combination with estrogen as oral contraceptive.
Background
Norgestimate was first described in the literature in 1977. It was developed by Ortho Pharmaceutical Corporation as part of an effort to develop new hormonal contraceptives with reduced adverse effects. It is commonly formulated with ethinylestradiol as a combined oral contraceptive that can also be used to treat moderate acne vulgaris.
Norgestimate was granted FDA approval on 29 December 1989.
Indications
Norgestimate is formulated with ethinylestradiol as a combined oral contraceptive. It can also be given with low dose ethinylestradiol for contraception as well as the treatment of moderate acne vulgaris in women ≥15 years old.
What are the applications of Application
Norgestimate is a progestogen
Definition
ChEBI: Norgestimate is a steroid ester, a ketoxime and a terminal acetylenic compound. It has a role as a contraceptive drug, a progestin and a synthetic oral contraceptive.
Manufacturing Process
A solution of 4.5 g of D-17β-acetoxy-13β-ethyl-17α-ethynyl-gon-4-en-3-one in 15 ml of pyridine and 2.0 g of hydroxylamine hydrochloride hydroxylamine hydrochloride is heated on a steam bath for 45 min. It is then cooled and poured into a large amount of ice-water, after which the solid which is thus produced is filtered off and air dried. Recrystallization from methylene chloride-ethanol gives D-17β-acetoxy-13α-ethyl-17α-ethynyl-gon-4-en-one oxime, m.p. 214-218°C; [α]D25 = +41°.
brand name
CILEST
Therapeutic Function
Progestin
General Description
Norgestimate, (17α)-17-acetyloxy-13-ethyl-18,19-dinor-pregn-4-en-20yn-3-one oxime, is a 19-nortestosterone, 3-oxime prodrug that is orally active andused with an estrogen in oral contraceptive products. It hasminimal androgenic action. Norgestimate is metabolized to17-deacetylnorgestimate (norelgestromin) and norgestrel,which provide the progestational action.
Pharmacokinetics
Norgestimate is a progestin that suppresses ovulation for contraception and reduces free testosterone to treat moderate acne vulgaris. The therapeutic index is wide as overdoses are rare. Patients should be counselled regarding the risk of vascular problems, liver disease, hypertension, metabolic effects, headaches, and bleeding irregularities.
in vitro
norgestimate was found that, unlike other 19-nortestosterone derivatives, showed high selectivity for the progesterone receptor and low androgenic activity. moreover, norgestimate and its main active metabolite norelgestromin could not bind to or occupy sex hormone-binding globulin [1].
in vivo
the androgenic and the progestational activity of norgestimate were compared in two animal studies. it was found the difference in the pharmacological response in norgestimate treated rats was equivalent to the difference in the exposure of the animals to either directly administered or metabolically derived levonorgestrel [2].
Metabolism
Norgestimate is rapidly deacetylated to the active 17-desacetylnorgestimate, which is deoximated to the active norgestrel. 17-desacetylnorgestimate is metabolized to a number of undefined hydroxylated metabolites, mainly by CYP3A4 and to a lesser extend by CYP2B6 and CYP2C9. Norgestrel is O-glucuronidated by UGT1A1 or oxidized to a number of undefined hydroxylated metabolites by CYP3A4.
Metabolism
Norgestimate is considered to be a pro-proges tin (prodrug), because it rapidly undergoes a two-s tep m etabolic transformation to form two active products, norelgestromine (levonorgestrel 3-oxime) and levonorgestrel. Deacetylation occurs in the intestine and liver, whereas convers ion of the 3-oxime to the corresponding ketone occurs primarily in the liver. Unlike the other progestins mentioned, norgestimate and its metabolites are not bound to SHBG.
References
[1] thomas l. lemke; david a. williams (2008). foye's principles of medicinal chemistry. lippincott williams & wilkins. pp. 1316–. isbn 978-0-7817-6879-5.
[2] kuhnz w, beier s. comparative progestational and androgenic activity of norgestimate and levonorgestrel in the rat. contraception. 1994 mar;49(3):275-89.
[3] https://en. wikipedia.org/wiki/norgestimate
Properties of Norgestimate
| Melting point: | 216°C |
| Boiling point: | 497.9±45.0 °C(Predicted) |
| alpha | D25 +110° |
| Density | 1.22±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO: soluble5mg/mL (clear solution) |
| form | powder |
| pka | 12.21±0.60(Predicted) |
| color | white to beige |
| optical activity | [α]/D 43±3°, c = 1 in chloroform |
| BRN | 6440219 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 35189-28-7 |
| EPA Substance Registry System | 18,19-Dinorpregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-, 3-oxime, (17.alpha.)- (35189-28-7) |
Safety information for Norgestimate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Norgestimate
Norgestimate manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran




You may like
-
 Norgestimate CAS 35189-28-7View Details
Norgestimate CAS 35189-28-7View Details
35189-28-7 -
 Norgestimate CAS 35189-28-7View Details
Norgestimate CAS 35189-28-7View Details
35189-28-7 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1