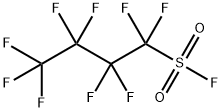
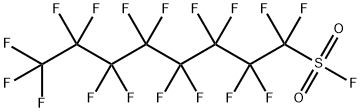
Nonafluorobutanesulfonyl fluoride
Synonym(s):NfF;Nonafluorobutanesulfonyl fluoride
- CAS NO.:375-72-4
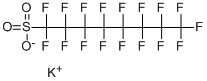
- Empirical Formula: C4F10O2S
- Molecular Weight: 302.09
- MDL number: MFCD00007422
- EINECS: 206-792-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is Nonafluorobutanesulfonyl fluoride?
Description
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonyl fluoride (NfF) also called Nonafluorobutanesulfonyl fluoride is a versatile compound in organic synthesis. It can be used as a fluoride source for the nucleophilic introduction of fluorine, but it is also frequently applied as sulfonylation reagent generating intermediates with strong electron withdrawing perfluorinated alkyl substituents.
Chemical properties
Nonafluorobutanesulfonyl fluoride is a colorless, volatile liquid that is immiscible with water but soluble in common organic solvents. It is prepared by the electrochemical fluorination of sulfolane.
The Uses of Nonafluorobutanesulfonyl fluoride
Benzynes were generated from o-(trimethylsilyl) phenols using nonafluorobutanesulfonyl fluoride (NfF) by a domino process.Nonafluorobutanesulfonyl fluoride (nonaflyl fluoride, C 4 F 9 SO 2 F, NfF) is the most widely used commercially available reagent for the synthesis of nonaflates.
The Uses of Nonafluorobutanesulfonyl fluoride
Nonafluorobutanesulfonyl fluoride can be used as a general-purpose diazotransfer reagent with high efficiency, wide substrate range, good shelf stability, relatively low cost, and easy purification. It is a favourable alternative to other commonly used diazotransfer reagents[1]. In addition, Nonafluorobutanesulfonyl fluoride was used in the formation of a new dehydrated glycosylation scheme. In contrast to the main classical glycosylation reactions, this scheme glycosylation reaction is carried out under mildly alkaline conditions. In the absence of an external acceptor, the glycosyl hemiacetal undergoes self-condensation, providing the corresponding symmetric 1,1'- disaccharide in high yield[2].
What are the applications of Application
Perfluoro-1-butanesulfonyl fluoride (NfF) reacts with alcohols, including phenols, to yield nonafluorobutanesulfonate esters (nonaflates). Nonaflates can be used as electrophiles in several palladium-catalyzed cross coupling reactions and in Buchwald-Hartwig amination. NfF can also be used to prepare aryl nonaflates by reacting with corresponding aryloxysilanes in the presence of fluoride ion.
Synthesis
NfF(Nonafluorobutanesulfonyl fluoride) is produced on industrial scale by anodic fluorination of sulfolene (1, Scheme 1),3 therefore it is a fairly cheap reagent and commercially available from several suppliers. The compound is bench-stable and storable for years, nontoxic and easy to handle.
storage
Store in amber colored bottles protected from light; for prolonged storage, PVC bottles are recommended.
References
[1] JOSE LUIS CHIARA; José R S. Synthesis of α-Diazo Carbonyl Compounds with the Shelf-Stable Diazo Transfer Reagent Nonafluorobutanesulfonyl Azide[J]. Advanced Synthesis & Catalysis, 2011. DOI:10.1002/adsc.201000846.
[2] YU TANG; Biao Y; D Prabhakar Reddy. A dehydrative glycosylation protocol mediated by nonafluorobutanesulfonyl fluoride (NfF)[J]. Tetrahedron, 2021. DOI:10.1016/j.tet.2020.131800.
Properties of Nonafluorobutanesulfonyl fluoride
| Melting point: | -110°C |
| Boiling point: | 64 °C (lit.) |
| Density | 1.682 g/mL at 25 °C (lit.) |
| vapor pressure | 16.665kPa at 20℃ |
| refractive index | n |
| Flash point: | 65-66°C |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Sparingly), Methanol (Sparingly) |
| form | Liquid |
| color | Colorless to yellow |
| Specific Gravity | 1.682 |
| Water Solubility | Hydrolyses in water. |
| Sensitive | Moisture Sensitive |
| BRN | 1813589 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 375-72-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Nonafluorobutanesulfonyl fluoride(375-72-4) |
| EPA Substance Registry System | 1-Butanesulfonyl fluoride, 1,1,2,2,3,3,4,4,4-nonafluoro- (375-72-4) |
Safety information for Nonafluorobutanesulfonyl fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Nonafluorobutanesulfonyl fluoride
| InChIKey | LUYQYZLEHLTPBH-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Perfluoro-1-butanesulfonyl fluoride, 96% CAS 375-72-4View Details
Perfluoro-1-butanesulfonyl fluoride, 96% CAS 375-72-4View Details
375-72-4 -
 Perfluoro-1-butanesulfonyl Fluoride CAS 375-72-4View Details
Perfluoro-1-butanesulfonyl Fluoride CAS 375-72-4View Details
375-72-4 -
 Nonafluorobutanesulfonyl fluoride 98% (GC) CAS 375-72-4View Details
Nonafluorobutanesulfonyl fluoride 98% (GC) CAS 375-72-4View Details
375-72-4 -
 Nonafluorobutanesulfonyl fluoride 96% CAS 375-72-4View Details
Nonafluorobutanesulfonyl fluoride 96% CAS 375-72-4View Details
375-72-4 -
 Perfluoro-1-butanesulfonyl fluoride CAS 375-72-4View Details
Perfluoro-1-butanesulfonyl fluoride CAS 375-72-4View Details
375-72-4 -
 375-72-4 Perfluorobutanesulfonyl fluoride 98%View Details
375-72-4 Perfluorobutanesulfonyl fluoride 98%View Details
375-72-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1