N,N-DiMethylMethyleneaMMoniuM Iodide
Synonym(s):N,N-Dimethylmethyleneammonium iodide;Eschenmoser’s salt
- CAS NO.:33797-51-2
- Empirical Formula: C3H8IN
- Molecular Weight: 185.01
- MDL number: MFCD00011810
- EINECS: 251-680-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-15 16:24:08
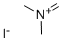
What is N,N-DiMethylMethyleneaMMoniuM Iodide?
Description
N, N-Dimethylmethyleneammonium iodide (DMMIA) is a versatile quaternary ammonium compound characterized by its unique chemical structure and widely utilized in scientific research. This colorless salt readily dissolves in water and has a melting point of 197°C. DMMIA is a crucial reagent in various laboratory experiments, contributing significantly to synthesis processes. Moreover, it finds application in biochemical studies, showcasing diverse biochemical and physiological effects. Its importance in scientific research is evident, as it serves as a reagent for organic synthesis, a catalyst for alcohol oxidation, and a key component in producing heterocyclic compounds. Additionally, it is utilized in synthesizing surfactants and other amphiphilic molecules. Furthermore, DMMIA aids in studying enzyme kinetics by effectively inhibiting certain enzyme activities.
Chemical properties
CREAM TO YELLOW CRYSTALLINE POWDER
The Uses of N,N-DiMethylMethyleneaMMoniuM Iodide
In organic synthesis.
The Uses of N,N-DiMethylMethyleneaMMoniuM Iodide
(N,N-Dimethyl)methyleneammonium iodide is a useful reagent for many synthetic applications like aminomethylation. It is also essential for the conversion of ketones to α, β-unsaturated enones. It is utilized to prepare derivatives of the type RCH2N(CH3)2. It is extensively used in medicines like painkillers and sedatives- Tramadol hydrochloride intermediate viz. 2-dimethylamine methylcyclohexanone. It is also employed in reactive dyes, synthesized spices. It plays an essential role in Mannich reactions.
General Description
N,N-Dimethylmethyleneiminium iodide is useful reagent for many synthetic applications, especially aminomethylation and conversion of ketones to α,β?unsaturated enones.
Synthesis
The mixture of trimethylamine, diiodomethane, dioxane and absolute ethanol was placed in the dark to react at room temperature for 100 h, the resulting crystals were collected by filtration, washed with ethanol and ether in turn, and dried under vacuum at 70 °C to obtain iodomethyl trimethylMethylammonium iodide. It was heated with sulfolane and kept at 160 °C for 12 min. The precipitated crystals were collected by filtration, washed with carbon tetrachloride, and vacuum dried at 50 °C to obtain N,N-DiMethylMethyleneaMMoniuM Iodide.
Properties of N,N-DiMethylMethyleneaMMoniuM Iodide
| Melting point: | 219 °C (dec.)(lit.) |
| Density | 1.7217 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Soluble in dimethylformamide. Partially soluble in acetonitrile, tetrahydrofuran and dichloromethane. |
| form | Crystalline Powder |
| color | Cream to yellow |
| Sensitive | Moisture & Light Sensitive |
| Merck | 14,3251 |
| BRN | 3594966 |
| InChI | InChI=1S/C3H8N.HI/c1-4(2)3;/h1H2,2-3H3;1H/q+1;/p-1 |
| CAS DataBase Reference | 33797-51-2(CAS DataBase Reference) |
Safety information for N,N-DiMethylMethyleneaMMoniuM Iodide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N,N-DiMethylMethyleneaMMoniuM Iodide
| InChIKey | VVDUZZGYBOWDSQ-UHFFFAOYSA-M |
| SMILES | [N+](=C)(C)C.[I-] |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N-(([(4-CHLOROANILINO)(METHYLSULFANYL)METHYLENE]AMINO)METHYLENE)-N-METHYLMETHANAMINIUM IODIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6360638.gif)

You may like
-
 Eschenmoser's salt 97% CAS 33797-51-2View Details
Eschenmoser's salt 97% CAS 33797-51-2View Details
33797-51-2 -
 N,N-Dimethylmethyleneiminium iodide 98.00% CAS 33797-51-2View Details
N,N-Dimethylmethyleneiminium iodide 98.00% CAS 33797-51-2View Details
33797-51-2 -
 N,N-Dimethylmethyleneammonium Iodide CAS 33797-51-2View Details
N,N-Dimethylmethyleneammonium Iodide CAS 33797-51-2View Details
33797-51-2 -
 N,N-Dimethylmethyleneiminium iodide CAS 33797-51-2View Details
N,N-Dimethylmethyleneiminium iodide CAS 33797-51-2View Details
33797-51-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1