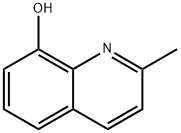
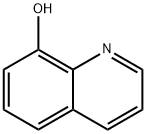
Nitroxoline
Synonym(s):5-Nitro-8-quinolinol
- CAS NO.:4008-48-4
- Empirical Formula: C9H6N2O3
- Molecular Weight: 190.16
- MDL number: MFCD00006791
- EINECS: 223-662-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04

What is Nitroxoline?
Description
Nitroxoline is an 8-hydroxyquinoline that has diverse biological activities, including antibacterial, antiproliferative, and bromodomain interaction-inhibiting properties. Nitroxoline is active against the bacteria E. coli, S. aureus, E. faecalis, K. pneumoniae, and P. mirabilis in vitro (MIC90s = 4, 4, 8, 8, and 8 mg/L, respectively). It also inhibits biofilm formation of certain strains of multidrug-resistant (MDR) A. baumannii and P. aeruginosa, as well as methicillin-resistant S. aureus (MRSA) and S. epidermidis (MRSE) with minimum biofilm eradication concentration (MBEC) values of 46.9, 1,500, 188, and 125 μM, respectively. Nitroxoline inhibits the growth of human U87 and U251 glioma, A549 lung, and PC3 prostate cancer cells (IC50s = 50, 6, 38, and 23 μg/ml, respectively). In vivo, it reduces tumor growth in a PTEN- and KRAS-driven glioma mouse model when administered at a dose of 80 mg/kg per day. Nitroxoline also inhibits the interaction between the first bromodomain of bromodomain-containing protein 4 (BRD4) with acetylated histone H4 with an IC50 value of 0.98 μM.
Chemical properties
ochre-yellow to brownish crystalline powder
The Uses of Nitroxoline
antibacterial
The Uses of Nitroxoline
5-Nitro-8-hydroxyquinoline is an antimicrobial agent used for the treatment of urinary tract infection. 5-Nitro-8-hydroxyquinoline is also a potent and reversible inhibitor of cathepsin B. 5-Nitro-8-h ydroxyquinoline has been shown to have anti-cancer activity.
The Uses of Nitroxoline
8-Hydroxy-5-nitroquinoline was used in the synthesis of novel CO2-soluble 8-hydroxyquinoline chelating agents.
What are the applications of Application
8-Hydroxy-5-nitroquinoline is used in the synthesis of novel CO2-soluble 8-hydroxyquinoline chelating agents
Definition
ChEBI: A monohydroxyquinoline in which the hydroxy group is positioned at C-8 with a nitro group trans to it at C-5.
brand name
5-nitrok;Dovenix;Entercol;Enterocol;Isinok;Nicene;Nikinol;Nikopet;Noxine;Trodax;Uro-coli.
World Health Organization (WHO)
Nitroxoline, a urinary antiseptic, was introduced in the mid-1960s. By the early 1970s long-term animal studies revealed the development of cataracts in rats and, although no serious adverse effects had been reported in man, the drug was withdrawn in at least two countries. Preparations containing nitroxoline remain widely available.
Biochem/physiol Actions
8-Hydroxy-5-nitroquinoline is an effective anti-microbial and anti-cancer agent. It is an effective drug for the treatment of urinary tract infections due to gram negative bacilli.
in vitro
the machnistic study showed that nitroxoline, in the treatment of acute or recurrent urinary tract infections caused by escherichia coli, could be decreased in the presence of mg2+ and mn2+ but not ca2+. moreover, with the divalent metal ions, a shift in the nitroxoline a448 indicated the formation of drug-ion complexes and a clear correlation was observed between the chelating property and antibacterial activity of nitroxolinet. in addition, it was found that the uptake was energy-independent and with biphasic kinetics: a rapid cell association phase and then a slower increase of cell- nitroxoline association [1].
in vivo
previous animal study showed that nitroxoline suspension with tween-80 in a could decrease the tone of the rat and guinea-pig ileum and diminish their peristalsis. moreover, when administered orally in a dose 50 mg/kg to rats, nitroxoline was able to inhibit the agar-, serotonin-, as well as carrageenin-induced edemas of the rat paws without affecting the response to subplantar histamine injection [2].
References
[1] pelletier c,prognon p,bourlioux p. roles of divalent cations and ph in mechanism of action of nitroxoline against escherichia coli strains. antimicrob agents chemother.1995 mar;39(3):707-13.
[2] zaks as,zil'ber al,kapitonenko ta. spasmolytic and anti-inflammatory activity of 8-hydroxyquinolines. farmakol toksikol.1984 sep-oct;47(5):44-7.
[3] lambert-zechovsky n,lévêque b,bingen e,pillion g,chapelle j,mathieu h. clinical study and effect of nitroxoline on fecal flora in childrenpathol biol (paris).1987 may;35(5):669-72.
Properties of Nitroxoline
| Melting point: | 181-183 °C(lit.) |
| Boiling point: | 325.64°C (rough estimate) |
| Density | 1.3907 (rough estimate) |
| refractive index | 1.5570 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | alcohol: very slightly soluble |
| form | powder to crystal |
| pka | 2.55±0.10(Predicted) |
| color | Light yellow to Brown to Dark green |
| Merck | 14,6655 |
| NIST Chemistry Reference | 8-Hydroxy-5-nitroquinoline(4008-48-4) |
Safety information for Nitroxoline
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nitroxoline
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 4008-48-4 Nitroxoline 98%View Details
4008-48-4 Nitroxoline 98%View Details
4008-48-4 -
 4008-48-4 98%View Details
4008-48-4 98%View Details
4008-48-4 -
 Nitroxoline 98%View Details
Nitroxoline 98%View Details
4008-48-4 -
 Nitroxoline 4008-48-4 98%View Details
Nitroxoline 4008-48-4 98%View Details
4008-48-4 -
 Nitroxoline 99%View Details
Nitroxoline 99%View Details -
 4008-48-4 NITROXOLINE 95-99%View Details
4008-48-4 NITROXOLINE 95-99%View Details
4008-48-4 -
 8-Hydroxy-5-nitroquinoline CAS 4008-48-4View Details
8-Hydroxy-5-nitroquinoline CAS 4008-48-4View Details
4008-48-4 -
 8-Hydroxy-5-nitroquinoline CAS 4008-48-4View Details
8-Hydroxy-5-nitroquinoline CAS 4008-48-4View Details
4008-48-4
