8-Hydroxyquinoline
Synonym(s):8-Hydroxyquinoline;8-Oxychinolin;8-Quinolinol;Oxine;Oxine, 8-Quinolinol
- CAS NO.:148-24-3
- Empirical Formula: C9H7NO
- Molecular Weight: 145.16
- MDL number: MFCD00006807
- EINECS: 205-711-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:32
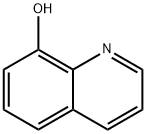
What is 8-Hydroxyquinoline?
Chemical properties
8-Hydroxyquinoline is a white to cream-colored crystal or crystalline powder that is insoluble in water or ether and freely soluble in ethanol, acetone, chloroform, benzene, and aqueous mineral acids. It readily forms stable metal chelates, which are soluble or precipitable in organic solvents, depending on the pH of the solution (Hollingshead, 1954).
Originator
Chinosol,Chinosolfabrik
The Uses of 8-Hydroxyquinoline
8-Hydroxyquinoline has a wide variety of uses, primarily due to its metal chelating properties. 8-Hydroxyquinoline and its salts, halogenated derivatives, and metal complexes have been utilized as analytical reagents (Hollingshead, 1954) and as antimicrobial agents in medicine, fungicides, and insecticides (Harvey, 1975). Additionally, it serves as a preservative in cosmetics and tobacco, a chemical intermediate in dye synthesis (IARC, 1977), and a precipitating reagent for uranium and other radioactive metals in nuclear power plant liquid waste effluent. It is also employed in nuclear medicine with indium-111 (Davis et al., 1978).
Background
Oxyquinoline, a heterocyclic phenol and derivative of quinoline, possesses antiseptic, disinfectant, and pesticide properties. It is also used as a stabilizer for hydrogen peroxide and is sometimes added to cosmetic products.
Indications
Oxyquinoline is used as a biocidal component of several over the counter products. These products are marketed for the purposes of inhibiting abnormal biological growth in the vagina and restoring natural pH.
Definition
ChEBI: 8-Hydroxyquinoline is a monohydroxyquinoline that is quinoline substituted by a hydroxy group at position 8. Its fungicidal properties are used for the control of grey mould on vines and tomatoes. It has a role as an antibacterial agent, an iron chelator, an antiseptic drug and an antifungal agrochemical. It derives from a hydride of a quinoline.
What are the applications of Application
8-Hydroxyquinoline may be used as a chelating ligand in the preparation of tris-(8-hydroxyquinoline)aluminum (Alq3), an organic electroluminescent compound used in organic light-emitting devices (OLEDs).
Preparation
8-Hydroxyquinoline is synthesized from o-aminophenol by cyclization reaction. Add glycerin into the acid-resistant reaction pot, slowly add concentrated sulfuric acid under stirring, and simultaneously add o-aminophenol and o-nitrophenol in sequence, and add 65% of the total oleum first. Heat up to 125°C, stop heating, naturally heat up to 140°C, and wait until the temperature returns to 136°C. The rest of the oleum was continued to be added, maintaining the temperature at 137°C. After adding acid, keep warm for 4 hours, cool to below 100°C, pump into an acid-resistant pot containing 10 times the amount of water (calculated by o-aminophenol), stir, heat to 75-80°C, use 30% sodium hydroxide The solution was neutralized to pH 7-7.2. The precipitate is released while hot, cooled into a block, and sublimed under reduced pressure to obtain the finished product of 8-hydroxyquinoline.
brand name
Aci-jel;Benzease;Chinosol;Cp-cap;Dermacid;Dermoplast;Fennosan h 30;Heriat;Hydroxybenoxopyridine;Medicone derma-hc;Oxykin;Oxyquinoline-rhp;Pedivol;Phenopyridine;Preconsol;Quinoderm;Quinoped;Quinophenol;Recta medicone-hc;Semori;Serohinol;Serorhinol;Superol;Trimo-san;Triva douch powder;Triva jel.
Therapeutic Function
Antiseptic
World Health Organization (WHO)
Halogenated hydroxyquinoline is structurally related to clioquinol. See WHO comment for clioquinol. (Reference: (WHODI) WHO Drug Information, 77.1, 9, 1977)
General Description
White to off-white or faintly yellow crystalline powder. Phenolic odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
8-Hydroxyquinoline darkens on exposure to light. 8-Hydroxyquinoline readily forms stable metal chelates. 8-Hydroxyquinoline is incompatible with strong oxidizers. 8-Hydroxyquinoline is also incompatible with many metal ions.
Hazard
Toxic by ingestion. Questionable carcinogen.
Biochem/physiol Actions
8-Hydroxyquinoline is a RNA synthesis inhibitor that acts as a fungicide against Trichophyton mentagrophytes, Myrothecium verrucaria, and Trichoderma viride. The antifungal mechanism of action is not clear but appears to be structurally related.
Pharmacokinetics
Oxyquinoline acts as a biocide to eliminate bacteria and fungi .
Clinical Use
8-Hydroxyquinoline (8HQ) is an intermediate of halogenated quinoline anti-amebic drugs, including quiniodoform, clioquinoline, diiodoquinoline and the like. These drugs exert anti-amebic effects by inhibiting intestinal commensal bacteria, and are effective against amoebic dysentery, but have no effect on extra-intestinal amoebic parasites.8HQ is a small planar molecule with a lipophilic effect and a metal chelating ability. As a result, 8HQ and its derivatives hold medicinal properties such as antineurodegenerative, anticancer, antioxidant, antimicrobial, anti-inflammatory, and antidiabetic activities.
Safety Profile
8-Hydroxyquinoline (8-HQ) may be a skin irritant in man. Hair depigmentation was seen in mice treated dermally. Dilute solutions were slightly irritating to the eyes of rabbits. In cases of human poisoning (by ingestion or by the administration of an enema containing 8-HQ or its sulphate), the kidney, liver and blood were the principal sites of toxic attack. Comprehensive studies involving repeated oral administration of 8-HQ to rodents failed to identify any particular sites for toxic attack and provided no convincing evidence of carcinogenicity. 8-HQ induced chromosomal damage in mammalian cells in culture (including human cells), but gave conflicting results in mice treated intraperitoneally. Both 8-HQ and its sulphate have induced mutagenicity in Ames bacterial tests and there was some evidence of mutagenic activity in mammalian cells treated in culture.
Metabolism
In the urine, 60% of the dose is excreted as glucuronide conjugates and 23% of the dose as sulfate conjugates . In the bile, 9% of the total dose is found as glucuronide conjugates.
Purification Methods
Crystallise oxine from hot EtOH, acetone, pet ether (b 60-80o) or water. Crude oxine can be purified by precipitation of copper oxinate, followed by liberation of free oxine with H2S or by steam distillation after acidification with H2SO4. Store it in the dark. It forms complexes with many metals. [Manske et al. Can J Research 27F 359 1949, Phillips Chem Rev 56 271 1956, Beilstein 21 III/IV 1135, 21/3 V 252.]
Properties of 8-Hydroxyquinoline
| Melting point: | 70-73 °C(lit.) |
| Boiling point: | 267 °C752 mm Hg(lit.) |
| Density | 1.0340 |
| vapor pressure | 0.221Pa at 25℃ |
| refractive index | 1.4500 (estimate) |
| Flash point: | 267°C |
| storage temp. | Store below +30°C. |
| solubility | 0.56g/l |
| pka | 5.017(at 20℃) |
| form | Liquid |
| color | White to pale yellow or light beige |
| Water Solubility | INSOLUBLE |
| Sensitive | Light Sensitive |
| Merck | 14,4843 |
| BRN | 114512 |
| CAS DataBase Reference | 148-24-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 8-Quinolinol(148-24-3) |
| IARC | 3 (Vol. 13, Sup 7) 1987 |
| EPA Substance Registry System | 8-Quinolinol (148-24-3) |
Safety information for 8-Hydroxyquinoline
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 8-Hydroxyquinoline
| InChIKey | MCJGNVYPOGVAJF-UHFFFAOYSA-N |
8-Hydroxyquinoline manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 8-Hydroxyquinoline 99%View Details
8-Hydroxyquinoline 99%View Details -
 8-hydroxyquinoline 98%View Details
8-hydroxyquinoline 98%View Details -
 8-Hydroxyquinoline CAS 148-24-3View Details
8-Hydroxyquinoline CAS 148-24-3View Details
148-24-3 -
 8-Hydroxyquinoline CAS 148-24-3View Details
8-Hydroxyquinoline CAS 148-24-3View Details
148-24-3 -
 8-Hydroxyquinoline CAS 148-24-3View Details
8-Hydroxyquinoline CAS 148-24-3View Details
148-24-3 -
 8-Hydroxyquinoline (8-Quinolinol) extrapure CAS 148-24-3View Details
8-Hydroxyquinoline (8-Quinolinol) extrapure CAS 148-24-3View Details
148-24-3 -
 8-HYDROXYQUINOLINE (Oxine) 99%View Details
8-HYDROXYQUINOLINE (Oxine) 99%View Details
148-24-3 -
 Commercial D1 10DO Hydroxy Quinoline, for IndustryView Details
Commercial D1 10DO Hydroxy Quinoline, for IndustryView Details
148-24-3
