Nitrous acid methyl ester
- CAS NO.:624-91-9
- Empirical Formula: CH3NO2
- Molecular Weight: 61.04
- EINECS: 210-870-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
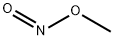
What is Nitrous acid methyl ester?
Chemical properties
A gas.
The Uses of Nitrous acid methyl ester
Synthesis of nitrile and nitroso esters.
General Description
A liquid or gas Boils at 10.4°F. Moderately toxic by inhalation. Narcotic in high concentrations. Used as a rocket propellant. Differs from nitromethane (H3CNO2).
Air & Water Reactions
Highly flammable. Somewhat soluble in water.
Reactivity Profile
Nitrous acid methyl ester is an oxidizing agent. A heat-sensitive explosive. [Lewis]. The presence of metal oxides increases the thermal sensitivity. May, if mixed with reducing agents including hydrides, sulfides and nitrides begin a vigorous reaction that culminates in a detonation. Reacts with inorganic bases to form explosive salts.
Hazard
Severe explosion risk when shocked orheated. Toxic by inhalation, narcotic.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Some may be toxic if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Silane will ignite spontaneously in air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Moderately toxic by inhalation. Mutation data reported. Narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame. A heat-sensitive explosive more powerful than ethyl nitrite. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Condense MeONO in a liquid nitrogen trap. Distil the greenish liquid under vacuum (preferably in a vacuum line), into the first trap containing dry Na2CO3 to free it from acid impurities then into further Na2CO3 and fused CaCl2 traps before collection at -78o. It has been distilled through columns that are surrounded by Et2O/Dri-Ice cooled to -30o. [Leermakers & Ramsperger J Am Chem Soc 54 1838 1932, Thompson & Purkis Trans Farad Soc 32 675 1936, Beilstein 1 H 284, 1 I 141, 1 II 273, 1 III 1201, 1 IV 1253.] CARCINOGEN.
Properties of Nitrous acid methyl ester
| Melting point: | -16.99°C |
| Boiling point: | -12°C |
| Density | 0.9910 |
| refractive index | 1.3418 (estimate) |
| EPA Substance Registry System | Methyl nitrite (624-91-9) |
Safety information for Nitrous acid methyl ester
Computed Descriptors for Nitrous acid methyl ester
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4