Nitrilotriacetic acid
Synonym(s):N,N-Bis(carboxymethyl)glycine;Nitrilotriacetic acid;NTA;Titriplex I;Tris(carboxymethyl)amine
- CAS NO.:139-13-9
- Empirical Formula: C6H9NO6
- Molecular Weight: 191.14
- MDL number: MFCD00004287
- EINECS: 205-355-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
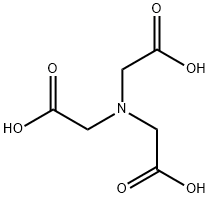
What is Nitrilotriacetic acid?
Chemical properties
Nitrilotriacetic acid, also known as NTA, is a white crystalline powder. It can dissolve in ammonia and alkali solutions while being slightly soluble in hot water. However, it is insoluble in water and most organic solvents. NTA can form mono-, di-, and tribasic salts, all of which are soluble in water. Combustible. 70% biodegradable.
The Uses of Nitrilotriacetic acid
Nitrilotriacetic acid is a chelating agent which forms coordination compounds with metal ions. Nitrilotriacetic acid is used in complexometric titrations and as well as for protein isolation and purif ication in the His-tag method.
The Uses of Nitrilotriacetic acid
Used in sequestration of metals; chelometric analysis.
The Uses of Nitrilotriacetic acid
Nitrilotriacetic acid is a chelating agent which forms coordination compounds with metal ions. Nitrilotriacetic acid is used in complexometric titrations and as well as for protein isolation and purification in the His-tag method. This compound is a contaminant of emerging concern (CECs).
What are the applications of Application
Nitrilotriacetic acid is acts as an excellent chelating agent
Definition
ChEBI: Nitrilotriacetic acid is a tricarboxylic acid and a NTA. It has a role as a nephrotoxic agent and a carcinogenic agent. It is a conjugate acid of a nitrilotriacetate(1-).
Preparation
The synthesis of nitrilotriacetic acid involves the following steps:
Chloroacetic acid is reacted with sodium hydroxide to produce sodium chloroacetate.
Sodium chloroacetate then reacts with ammonium chloride to form sodium aminotriacetate.
The resulting sodium aminotriacetate is acidified to obtain nitrilotriacetic acid.
Synthesis Reference(s)
The Journal of Organic Chemistry, 15, p. 46, 1950 DOI: 10.1021/jo01147a008
General Description
Odorless white solid. Sinks in and mixes with water.
Air & Water Reactions
Water Insoluble.
Reactivity Profile
Nitrilotriacetic acid is incompatible with strong oxidizers, aluminum, copper, copper alloy and nickel. Nitrilotriacetic acid is also incompatible with strong bases.
Hazard
Possible carcinogen.
Health Hazard
Toxicity and health hazard of these compounds are low. Contact with eyes causes irritation.
Fire Hazard
Flash point data for Nitrilotriacetic acid are not available; however, Nitrilotriacetic acid is probably combustible.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Nitrilotriacetic (NTA) has the ability to link with histidine side chain of proteins. It can be used in fluorescent labelling, hexahistidine (His6)-tagged proteins for purification and surface immobilization.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx,.
Potential Exposure
Nitrilotriacetic acid (NTA) was used as a phosphate replacement in laundry detergents in the late 1960s. In 1971, the use of NTA was discontinued. The possibility of resumed use arose in 1980. NTA is now used in laundry detergents in states where phosphates are banned. NTA is also used as a boiler feed-water additive at a maximum use level of 5 ppm of trisodium salt. Currently, the remaining nondetergent uses of NTA are for water treatment, textile treatment; metal plating and cleaning; and pulp and paper processing.
Carcinogenicity
Nitrilotriacetic acid is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it from water and dry it at 110o. [Beilstein 4 IV 2441.]
Incompatibilities
Dust may form explosive mixture with air. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Attacks aluminum, copper, copper alloy and nickel.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Nitrilotriacetic acid
| Melting point: | 245 °C (dec.)(lit.) |
| Boiling point: | 326.86°C (rough estimate) |
| Density | 1.5784 (rough estimate) |
| vapor pressure | 0-0Pa at 20-25℃ |
| refractive index | 1.4860 (estimate) |
| Flash point: | 100 °C |
| storage temp. | 2-8°C |
| solubility | 0.1 M NaOH: 0.1 M at 20 °C, clear, colorless |
| form | Powder |
| pka | 3.03, 3.07, 10.70(at 20℃) |
| color | White |
| Water Solubility | Insoluble. <0.01 g/100 mL at 23 ºC |
| λmax | λ: 260 nm Amax: 1.5 λ: 280 nm Amax: 0.05 |
| Merck | 14,6579 |
| BRN | 1710776 |
| CAS DataBase Reference | 139-13-9(CAS DataBase Reference) |
| IARC | 2B (Vol. 48, 73) 1999 |
| NIST Chemistry Reference | Nitrilotriacetic acid(139-13-9) |
| EPA Substance Registry System | Nitrilotriacetic acid (139-13-9) |
Safety information for Nitrilotriacetic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nitrilotriacetic acid
Nitrilotriacetic acid manufacturer
JSK Chemicals
New Alliance Dye Chem Pvt Ltd
Satyajit Chemicals Pvt Ltd
Bhakti Chemicals Pvt Ltd
I Chess Chemicals Pvt Ltd
ACI Industrial Organic PVT LTD
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 NTA acid 99%View Details
NTA acid 99%View Details -
 Nitrilotriacetic acid CAS 139-13-9View Details
Nitrilotriacetic acid CAS 139-13-9View Details
139-13-9 -
 NTA Acid CAS 139-13-9View Details
NTA Acid CAS 139-13-9View Details
139-13-9 -
 Nitrilotriacetic acid CAS 139-13-9View Details
Nitrilotriacetic acid CAS 139-13-9View Details
139-13-9 -
 Nitrilotriacetic acid, GR 99%+ CAS 139-13-9View Details
Nitrilotriacetic acid, GR 99%+ CAS 139-13-9View Details
139-13-9 -
 Nitrilotriacetic acid CAS 139-13-9View Details
Nitrilotriacetic acid CAS 139-13-9View Details
139-13-9 -
 Nitrilotriacetic acid 98% CAS 139-13-9View Details
Nitrilotriacetic acid 98% CAS 139-13-9View Details
139-13-9 -
 Nitrilotriacetic acid 99% AR/ACS CAS 139-13-9View Details
Nitrilotriacetic acid 99% AR/ACS CAS 139-13-9View Details
139-13-9