NIOBIUM
- CAS NO.:7440-03-1
- Empirical Formula: Nb
- Molecular Weight: 92.91
- MDL number: MFCD00011126
- EINECS: 231-113-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is NIOBIUM?
Chemical properties
shiny grey metallic solid
Chemical properties
Niobium was discovered by Charles Hatchett in 1801 and isolated by Christian Blomstrand of Sweden in 1964. Its name was given after the Greek mythological ?gure Niobe, the daughter of Tantalos; tantalum always was associated with niobium. For many years, the terms “niobium” and “columbium”wereusedinterchangeably;however,thename “niobium” was of?cially adopted by International Union of Pure and Applied Chemistry (IUPAC) in 1950. Niobium is not a very rare element; its crustal abundance is 24ppm, which is similar or greater than those of many common elements, such as lead or cobalt.
Niobium is a shiny white, soft, and malleable metal. The element is inert to HCl, HNO3, or aqua regia at room temperature, slightly soluble in HF, but is attacked by alkali hydroxides or oxidizing agents at all temperatures. In pure
form, niobium is ductile, unless it is allowed to associate at elevated temperatures with common gases such as N2,H 2, or O2. Thus, when processed, it must be placed in a protective environment
No data were found in the literature; however, it might be assumed that niobium and most of their compounds are odorless. Niobium pentachloride (NbCl5) has pungent odor, because it decomposes slowly when heated, with Cl2 formation. Niobium in the form of dust is moderately explosive when exposed to ?ame or by chemical reaction.
Physical properties
Niobium is a soft grayish-silvery metal that resembles fresh-cut steel. It is usually found inminerals with other related metals. It neither tarnishes nor oxidizes in air at room temperaturebecause of a thin coating of niobium oxide. It does readily oxidize at high temperatures(above 200°C), particularly with oxygen and halogens (group 17). When alloyed with tin andaluminum, niobium has the property of superconductivity at 9.25 Kelvin degrees.
Its melting point is 2,468°C, its boiling point is 4,742°C, and its density is 8.57 g/cm3.
Isotopes
There are 49 isotopes of niobium, ranging from Nb-81 to Nb-113. All are radioactiveand made artificially except niobium-93, which is stable and makes up all of theelement’s natural existence in the Earth’s crust.
Origin of Name
Niobium is named after the Greek mythological figure Niobe who was the daughter of Tantalus. Tantalus was a Greek god whose name is the source of the word “tantalize,” which implies torture: he cut up his son to make soup for other gods.
Occurrence
Niobium is the 33rd most abundant element in the Earth’s crust and is considered rare.It does not exist as a free elemental metal in nature. Rather, it is found primarily in severalmineral ores known as columbite (Fe, Mn, Mg, and Nb with Ta) and pyrochlore [(Ca,Na)2Nb2O6 (O, OH, F)]. These ores are found in Canada and Brazil. Niobium and tantalum[(Fe, Mn)(Ta, Nb)2O6] are also products from tin mines in Malaysia and Nigeria. Niobium is a chemical “cousin” of tantalum and was originally purified by its separation through theprocess known as fractional crystallization (separation is accomplished as a result of the differentrates at which some elements crystallize) or by being dissolved in special solvents. Todaymost of the niobium metal is obtained from columbite and pyrochlore through a complicatedrefining process that ends with the production of niobium metal by electrolysis of moltenniobium potassium fluoride (K2NbF7).
History
Niobium was discovered in 1801 by Hatchett in an ore sent to England more that a century before by John Winthrop the Younger, first governor of Connecticut. The metal was first prepared in 1864 by Blomstrand, who reduced the chloride by heating it in a hydrogen atmosphere. The name niobium was adopted by the International Union of Pure and Applied Chemistry in 1950 after 100 years of controversy. Most leading chemical societies and government organizations refer to it by this name. Some metallurgists and commercial producers, however, still refer to the metal as “Niobium.” Niobium is found in niobite (or columbite), niobite-tantalite, pyrochlore, and euxenite. Large deposits of niobium have been found associated with carbonatites (carbon-silicate rocks), as a constituent of pyrochlore. Extensive ore reserves are found in Canada, Brazil, Congo-Kinshasa, Rwanda, and Australia. The metal can be isolated from tantalum, and prepared in several ways. It is a shiny, white, soft, and ductile metal, and takes on a bluish cast when exposed to air at room temperatures for a long time. The metal starts to oxidize in air at 200°C, and when processed at even moderate temperatures must be placed in a protective atmosphere. It is used in arc-welding rods for stabilized grades of stainless steel. Thousands of pounds of niobium have been used in advanced air frame systems such as were used in the Gemini space program. It has also found use in super-alloys for applications such as jet engine components, rocket subassemblies, and heat-resisting equipment. The element has superconductive properties; superconductive magnets have been made with Nb-Zr wire, which retains its superconductivity in strong magnetic fields. Natural niobium is composed of only one isotope, 93Nb. Forty-seven other isotopes and isomers of niobium are now recognized. Niobium metal (99.9% pure) is priced at about 50¢/g.
Characteristics
Some of niobium’s characteristics and properties resemble several other neighboring elementson the periodic table, making them, as well as niobium, difficult to identify. This isparticularly true for tantalum, which is located just below niobium on the periodic table.
Niobium is not attacked by cold acids but is very reactive with several hot acids such ashydrochloric, sulfuric, nitric, and phosphoric acids. It is ductile (can be drawn into wiresthrough a die) and malleable, which means it can be worked into different forms.
The Uses of NIOBIUM
In ferrous metallurgy: Ferroniobium (produced by silicon reduction of columbite) is used to alloy stainless steels and metals for welding rods. In niobium base alloys for high temperatures and nuclear reactions. Niobium has some use as a getter in electronic vacuum tubes.
The Uses of NIOBIUM
Refined niobium metal is most useful as an alloy with other metals. It is used to producespecial stainless steel alloys, to make high-temperature magnets, as special metals for rocketsand missiles, and for high- and low-temperature–resistant ceramics. Stainless steel that hasbeen combined with niobium is less likely to break down under very high temperatures.This physical attribute is ideal for construction of both land- and sea-based nuclear reactors.Niobium has special cryogenic properties. It can withstand very cold temperatures, whichimproves its ability to conduct electricity. This characteristic makes it an excellent metal forlow-temperature electrical superconductors.
Niobium alloyed with germanium becomes a superconductor of electricity that does notlose its superconductivity at 23.2° Kelvin as large amounts of electrical current are passedthrough it, as do some other superconductive alloys. In the pure metallic state, niobium wiresare also superconductors when the temperatures are reduced to near absolute zero (–273°C).Niobium alloys are also used to make superconductive magnets as well as jewelry.
.
The Uses of NIOBIUM
Niobium sputtering target is used in high temperature engineering products. It is also used as an alloying agent for certain steels where it greatly improves the strength of the resulting material. It is also finds applications in atomic reactors due to its corrosion resistance and, when combined with either tin (Nb3Sn) or zirconium, it has a high degree of superconductivity.
Definition
The name niobium is officially approved by chemical authorities, but columbium is still used chiefly by metallurgists. Metallic element, atomic number 41, group VB of the periodic table, aw 92.9064, valences of 2, 3, 4, 5; no stable isotopes
Definition
A soft silvery transition element used in welding, special steels, and nuclear reactor work. Symbol: Nb; m.p. 2468°C; b.p. 4742°C; r.d. 8.570 (20°C); p.n. 41; r.a.m. 92.90638.
Definition
Columbium: a former name for theelement niobium.
Definition
niobium: Symbol Nb. A soft ductilegrey-blue metallic transition element;a.n. 41; r.a.m. 92.91; r.d. 8.57;m.p. 2468°C; b.p. 4742°C. It occurs inseveral minerals, including niobite(Fe(NbO3)2), and is extracted by severalmethods including reduction ofthe complex fluoride K2NbF7 usingsodium. It is used in special steelsand in welded joints (to increasestrength). Niobium–zirconium alloysare used in superconductors. Chemically,the element combines with thehalogens and oxidizes in air at 200°C.It forms a number of compounds andcomplexes with the metal in oxidationstates 2, 3, or 5. The elementwas discovered by Charles Hatchett(c. 1765–1847) in 1801 and first isolatedby Christian Blomstrand(1826–97) in 1864. Formerly, it wascalled columbium.
Production Methods
Theextractingandre?ningprocessesforniobiumfromore are extremely complex and consist of a series of operations, starting from upgrading the ores by concentration. Disruption of the niobium-containing matrix is then performed by an ore-opening procedure with hot HF or fusion with alkali ?uxes. The next steps include pure niobium compound preparation and reduction to metallic niobium, followed by re?ning, consolidation, and fabrication of the metal. Niobium is so closely associated with tantalum that they must be separated by fractional crystallization or by solvent extraction before puri?cation.
Hazard
Niobium is not considered reactive at normal room temperatures. However, it is toxic in itsphysical forms as dust, powder, shavings, and vapors, and it is carcinogenic if inhaled or ingested.
Carcinogenicity
No evidence was found that niobiumiscarcinogenic.Indeed,therearesomestudiessuggesting its antitumor activity. In the mouse study of Schroeder et al., occurrence of 23.6% of tumors in the niobiumtreated group (5–6.62ppm niobium in drinking water and diet for a lifetime) versus 34.8% for the controls was documented.
Properties of NIOBIUM
| Melting point: | 2468 °C (lit.) |
| Boiling point: | 4742 °C (lit.) |
| Density | 8.57 g/mL at 25 °C (lit.) |
| storage temp. | -20°C |
| solubility | insoluble in acid solutions |
| form | wire |
| color | Silver-gray |
| Specific Gravity | 8.57 |
| Resistivity | 13-16 μΩ-cm, 20°C |
| Water Solubility | Insoluble in water. |
| Merck | 13,6584 |
| Exposure limits | ACGIH: TWA 0.5 ppm(2.5 mg/m3); Ceiling 2 ppm (Skin) OSHA: TWA 3 ppm NIOSH: IDLH 30 ppm(250 mg/m3); TWA 3 ppm(2.5 mg/m3); Ceiling 6 ppm(5 mg/m3) |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents, halogens, oxygen. |
| CAS DataBase Reference | 7440-03-1(CAS DataBase Reference) |
| EPA Substance Registry System | Niobium (7440-03-1) |
Safety information for NIOBIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for NIOBIUM
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
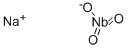

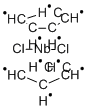
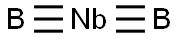

You may like
-
 Niobium CAS 7440-03-1View Details
Niobium CAS 7440-03-1View Details
7440-03-1 -
 Niobium CAS 7440-03-1View Details
Niobium CAS 7440-03-1View Details
7440-03-1 -
 Niobium CAS 7440-03-1View Details
Niobium CAS 7440-03-1View Details
7440-03-1 -
 Niobium foil, 0.25mm (0.01 in.) thick, Annealed CAS 7440-03-1View Details
Niobium foil, 0.25mm (0.01 in.) thick, Annealed CAS 7440-03-1View Details
7440-03-1 -
 Niobium foil, 0.25mm (0.01 in.) thick, Annealed CAS 7440-03-1View Details
Niobium foil, 0.25mm (0.01 in.) thick, Annealed CAS 7440-03-1View Details
7440-03-1 -
 Niobium CAS 7440-03-1View Details
Niobium CAS 7440-03-1View Details
7440-03-1 -
 Niobium wire, 0.25mm (0.01 in.) dia. CAS 7440-03-1View Details
Niobium wire, 0.25mm (0.01 in.) dia. CAS 7440-03-1View Details
7440-03-1 -
 Niobium wire, 0.25mm (0.01 in.) dia. CAS 7440-03-1View Details
Niobium wire, 0.25mm (0.01 in.) dia. CAS 7440-03-1View Details
7440-03-1
