NCX 116
- CAS NO.:860005-21-6
- Empirical Formula: C27H41NO8
- Molecular Weight: 507.62
- MDL number: MFCD30532608
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:20:40
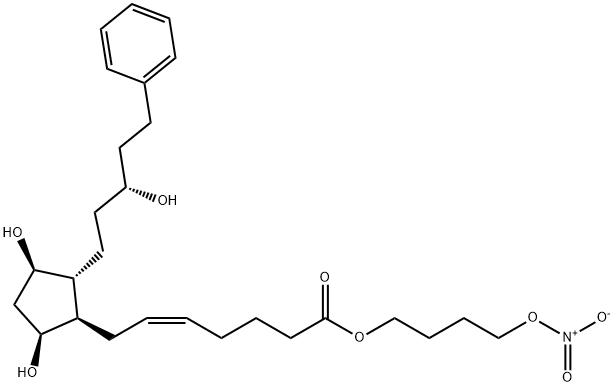
What is NCX 116?
Absorption
In a study with 22 healthy subjects monitored for 28 days, there were no quantifiable plasma concentrations of latanoprostene bunod (Lower Limit Of Quantitation, LLOQ, of 10.0 pg/mL) or butanediol mononitrate (LLOQ of 200 pg/mL) post daily dose of one drop bilaterally in the morning on Day 1 and 28 .
The mean time of maximum plasma concentration (Tmax) for latanoprost acid was about 5 minutes post dosage on both Day 1 and 28 of therapy .
The mean maximum plasma concentrations (Cmax) of latanoprost acid (LLOQ of 30 pg/mL) were 59.1 pg/mL on Day 1 and 28, respectively .
Toxicity
There are no available human data for the use of latanoprost bunod during pregnancy to inform any drug associated risks. Use during pregnancy must consider whether any potential benefit to the patient will justify the risk presented to the fetus .
There are no data on the presence of latanoprost bunod in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding must be considered along with the mother's potential clinical need for latanoprost bunod and any possible risk to the breastfed infant .
Prolonged, continued use of latanoprost bunod 0.024% opthalmic solution is expected to cause increased pigmentation of the iris and eyelid. Such pigmentation is expected to increase for as long as the medication is used. Upon discontinuing the medication, although pigmentation changes of the eyelid tissue may likely reverse, pigmentation changes to the iris is likely permanent. Such pigmentation changes typically present as brown pigmentation spreading and increasing concentrically outward from the pupil. These changes may not be noticeable for several months to years. The long-term effects of increased pigmentation are not known and any prospective patients should be informed of this effect. While usage of the medication can continue in patients who do develop such pigmentation changes they should also be examined regularly .
Prolonged, continued use of this medication is also expected to cause changes involving the increased length, thickness, and number of eyelash hairs. These changes are usually reversible upon discontinuation of the medication.
Latanoprost bunod can cause or exacerbate existing intraocular inflammation (iritis or uveitis). Use with caution in patients with a history of or active intraocular inflammation.
As a prostaglanding analog, latanoprost bunod has the potential to cuase macular edema, including cystoid macular edema. Use with caution in aphakic patients, in pseudoaphakic patients with a torn posterior lens capsule, or in patients with with known risk factors for macular edema.
Bacterial keratitis or other eye infections are commonly associated with the use of opthalmic solution containers that have been inadvertently contaminated by patients who have a concurrent corneal disease or a disruption of the ocular epithelial surfaced.
Contact lenses should be removed prior to the use of latanoprost bunod because its benzalkonium chloride preservative can affect or alter contact lenses .
The most common adverse reactions obseved in patients treated with latanoprostene bunod during clinical trials were conjuctival hyperemica, eye irritation, eye pain, and installation site pain [FDA Lable].
The Uses of NCX 116
Latanoprostene Bunod is a derivative of Latanoprost and is used in the treatment of interocular pressure.
Background
Latanoprostene Bunod has been used in trials studying the treatment of Glaucoma, Ocular Hypertension, Open-Angle Glaucoma, Open Angle Glaucoma, and Intraocular Pressure.
As of November 2, 2017 the FDA approved Bausch + Lomb's Vyzulta (latanoprostene bunod opthalmic solution), 0.024% for the indication of reducing intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Latanoprostene bunod is the first prostaglandin analog with one of its metabolites being nitric oxide (NO). The novelty of this agent subsequently lies in the proposed dual mechanism of action that stems from both its prostaglandin F2-alpha analog latanoprost acid metabolite and its ability to donate NO for proposed tissue/cell relaxation effects.
In comparison, both latanoprost and latanoprostene bunod contain a latanoprost acid backbone. Conversely however, latanoprostene bunod integrates an NO-donating moiety in lieu of the isopropyl ester typically found in latanoprost.
Indications
Latanoprostene bunod opthalmic solution is indicated for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension .
Definition
ChEBI: Latanoprostene bunod is a prostanoid.
Pharmacokinetics
Upon applying an appropriate dose of latanoprost bunod, reduction in intraocular pressure begins approximately 1 to 3 hours later with a maximum intraocular pressure reduction effect demonstrated after 11 to 13 hours .
Metabolism
Upon topical administration at the ocular surface, latanoprostene bunod undergoes rapid carboxyl ester hydrolysis by endogenous corneal esterases into latanoprost acid and butanediol mononitrate .
After the latanoprost acid reaches the systemic circulation, it is largely metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites by way of fatty acid beta-oxidation .
The butanediol monohidrate undergoes further metabolism (reduction) to 1,4-butanediol and nitric oxide (NO). Furthermore, this 1,4-butanediol metabolite is further oxidized to succinic acid that is subsequently then primarily taken up as a component in the tricarboxylic acid (TCA) cycle in cellular aerobic respiration .
Properties of NCX 116
| Boiling point: | 658.3±55.0 °C(Predicted) |
| Density | 1.178±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -86°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| pka | 14.84±0.70(Predicted) |
| color | Colourless to Light Yellow to Thick |
| Stability: | Hygroscopic, Temperature Sensitive |
Safety information for NCX 116
Computed Descriptors for NCX 116
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
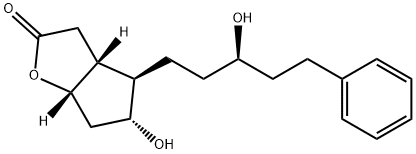
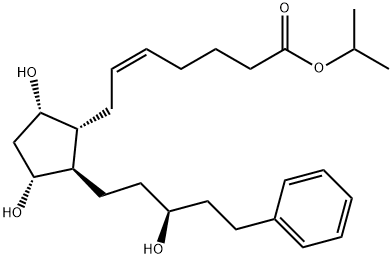
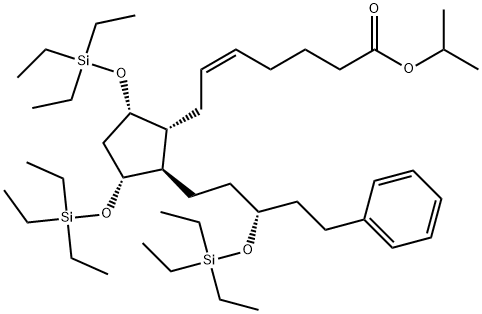
![(5Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-(3-oxo-5-phenylpentyl)cyclopentyl]-5-heptenoic Acid](https://img.chemicalbook.in/CAS/GIF/369585-22-8.gif)

You may like
-
 860005-21-6 Latanoprostene Bunod 99%View Details
860005-21-6 Latanoprostene Bunod 99%View Details
860005-21-6 -
 latanoprostene bunod 860005-21-6 99%View Details
latanoprostene bunod 860005-21-6 99%View Details
860005-21-6 -
 860005-21-6 latanoprostene bunod 98%View Details
860005-21-6 latanoprostene bunod 98%View Details
860005-21-6 -
 Latanoprostene Bunod APIView Details
Latanoprostene Bunod APIView Details
860005-21-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
