narciclasine
Synonym(s):Narciclasine;Lycoricidinol;(2S-(2-alpha,3-beta,4-beta,4a-beta))-3,4,4a,5-tetrahydro-2,3,4,7-tetrahydroxy-(1,3)Dioxolo(4,5-j)phenanthridin-6(2H)-one;3,4,4a,5-Tetrahydro-2,3,4,7-tetrahydroxy-(1,3)dioxolo(4,5-j)phenanthridin-6(2H)-one;Lycoricidin-A
- CAS NO.:29477-83-6
- Empirical Formula: C14H13NO7
- Molecular Weight: 307.26
- MDL number: MFCD01729949
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
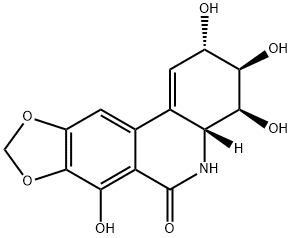
What is narciclasine?
Description
Narciclasine is a plant growth inhibitor that can be isolated from Narcissus bulbs. At 1 μM, it has been shown to induce apoptosis-mediated cytotoxicity in human cancer cells in vitro but not in normal fibroblasts. Narciclasine has been shown to regulate the Rho/Rho kinase/LIM kinase/cofilin signaling pathway by increasing GTPase RhoA activity, as well as inducing actin stress fiber formation in a RhoA-dependent manner.
Description
Narciclasine, also known as lycoricidinol, is an isocarbostyril alkaloid found in the Amaryllidaceae (amaryllis) family of flowering plants. One notable genus of the family is Narcissus, from which narciclasine gets its name. Daffodils and jonquils are members of the Narcissus genus.
In 1967, Giovanni Ceriotti, Luigi Spandrio, and Annivale Gazzaniga at Circolo Hospital (Busto Arsizio, Italy) isolated narciclasine from Narcissus varieties. At that time, it was known to inhibit cell division. By the early 2010s, narciclasine was an established antitumor agent.
In 2016, Robert Fürst at Goethe University (Frankfurt am Main, Germany) reviewed the antitumor and anti-inflammatory findings on narciclasine. At present, no clinical trials on narciclasine have been conducted on humans.
Narciclasine is sparingly soluble in water. But in 2003, George R. Petit and colleagues at Arizona State University (Tempe) synthesized a cyclic phosphate at two of the adjacent hydroxyl groups of narciclasine. The product, which they called narcistatin (CAS Reg. no. 496963-44-1), is much more water-soluble (4 g/L), making it a valuable prodrug for narciclasine.
Description
The bulbs of several Narcissus species contain this alkaloid which crystallizes in the form of pale yellow needles with a pronounced yellow-green fluorescence. It is best purified by recrystallization from either acetic acid or an aqueous mixture of methoxyethyl alcohol. It is dextrorotatory with [α]589 + 145° or [Qh64 + 983° (c 1.5, EtOH). The ultraviolet spectrum in neutral solution (EtOH) has absorption maxima at 252, 302 and 329 mil, while that in alkaline solution (0.01 N/NaOH) has the maxima at 219, 249,310 and 355 mil. The O-methyl ether forms shiny needles which also exhibit a strong blue fluorescence. The tri_x0002_acetate has been prepared as an amorphous, non-crystallizable substance. With FeC13 the alkaloid gives a violet colour.
The Uses of narciclasine
Narciclasine is reasonably abundant in some Narcissus spp. and has served as a very useful intermediate for synthetic conversion into (+)-pancratistatin and to conduct a series of structure–activity relationship studies . Bicolorine, another member of the narciclasine series, is an unusual, completely aromatized quaternary alkaloid with an N-methyl group.
The Uses of narciclasine
Narciclasine is an antiproliferative and pro-apoptotic inducer.
What are the applications of Application
Narciclasine is an antiproliferative and pro-apoptotic inducer
Definition
ChEBI: A natural product found in Narcissus pseudonarcissus.
storage
Store at -20°C
References
Ceriotti., Nature, 213,595 (1967)
Piozzi et al., Tetrahedron, 24, 1119 (1968)
Revised structure:
Mondon, Krohn., Tetrahedron Lett., 2123 (1970)
Savona, Piozzi, Marino., Chern. Cornrnun., 1006 (1970)
Absolute configuration:
Fuganti, Mazza.,J. Chern. Soc., Chern. Cornrnun., 239 (1972)
Crystal structure:
Immirzi, Fuganti., J. Chern. Soc., Chern. Cornrnun., 240 (1972)
Stereochemistry:
Mondon, Krohn., Tetrahedron Lett., 2085 (1972)
Properties of narciclasine
| Melting point: | 250-252℃ |
| Boiling point: | 447.77°C (rough estimate) |
| Density | 1.85±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: ≥10mg/mL |
| pka | 7.72±0.70(Predicted) |
| form | Solid |
| appearance | white to off-white powder |
| color | white to off-white |
Safety information for narciclasine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for narciclasine
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Narciclasine CAS 29477-83-6View Details
Narciclasine CAS 29477-83-6View Details
29477-83-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8