NAPHTHACENE
Synonym(s):2,3-Benzanthracene;Naphthacene;Tetracene
- CAS NO.:92-24-0
- Empirical Formula: C18H12
- Molecular Weight: 228.29
- MDL number: MFCD00003702
- EINECS: 202-138-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
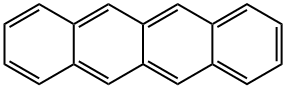
What is NAPHTHACENE?
Description
Tetracene is a four-ring polynuclear (i.e., polycyclic) aromatic hydrocarbon (PAH). It is the second member of the “acene” family of linearly arranged PAHs that begins with anthracene and continues to pentacene, hexacene, and beyond. The bright orange molecule is also known as naphthacene, 2,3-benzanthracene, and benz[b]anthracene.
Unlike many PAHs, tetracene is not carcinogenic. But it was studied for carcinogenicity as long ago as the 1930s. Early researchers were James W. Cook and co-workers at the Cancer Hospital of London and Alfred Winterstein and Karl Sch?n at the Kaiser Wilhelm Institute for Medical Research1 (Heidelberg, Germany).
Not much was done with tetracene until 2007, when researchers discovered that it is a semiconductor that was useful in organic field-effect transistors (OFETs) and organic light-emitting diodes (OLEDs). This year, electrical engineer Marc A. Baldo at MIT (Cambridge, MA) and colleagues there and at Princeton University (NJ) incorporated it into silicon solar cells (SSCs).
The maximum efficiency (conversion of sunlight into useful electricity) of single-layer SSCs is currently 29%. When the researchers added the semiconductor tetracene to SSCs, the cells’ efficiency increased to 35%. Through a complex process involving the emission of excitons from tetracene, the SSCs’ wavelength range widened, making the cells more efficient.
Other solar cell researchers consider the Baldo team’s findings to be a major breakthrough and intend to follow it up by incorporating them in their own research.
1. Now the Max Planck Institute for Medical Research.
Chemical properties
orange powder
The Uses of NAPHTHACENE
Naphthacene is a polycyclic aromatic hydrocarbon. Naphthacene is often used as a molecular organic semiconductor in organic field-effect transistors and organic light-emitting diodes. Naphthacene is a lso used in various applications involving the enhancement of photocurrent.
The Uses of NAPHTHACENE
p-Type organic conductor and OLED dopant. Sublimed grade for organic electronic device applications.
The Uses of NAPHTHACENE
Naphthacene is a polycyclic aromatic hydrocarbon. Naphthacene is often used as a molecular organic semiconductor in organic field-effect transistors and organic light-emitting diodes. Naphthacene is also used in various applications involving the enhancement of photocurrent.
Definition
ChEBI: An acene that consists of four ortho-fused benzene rings in a rectilinear arrangement.
General Description
Benz[b]anthracene is an ethylnylated acene that is a conducting polymer, which can be used as a donor material. It has a high photoluminescence and quantum yield that makes it a promising candidate in the development of single crystal electronic material.
Hazard
Explodes when shocked, reacts with oxidizing materials.
Purification Methods
Naphthacene crystallises in orange needles from EtOH, *C6H6 or toluene. Dissolve it in sodium-dried *benzene and pass it through a column of alumina. The eluent is evaporated under vacuum, and the chromatography is repeated using fresh *benzene. Finally, the naphthacene is sublimed in vacuo at 186o. [Martin & Ubblehode J Chem Soc 4948 1961, UV: Clar Chem Ber 65 517 1932, Clar Chem Ber 69 607 1936, IR: Cannon & Sutherland Spectrochim Acta 4 373 1951, Beilstein 5 H 718, 5 IV 2545.]
Properties of NAPHTHACENE
| Melting point: | >300 °C(lit.) |
| Boiling point: | 305.1°C (rough estimate) |
| Density | 1.35 |
| refractive index | 1.5500 (estimate) |
| solubility | insoluble |
| appearance | yellow-orange to red-orange crystals or powder |
| form | Crystalline Powder |
| color | Orange |
| Water Solubility | 1.507ug/L(25 ºC) |
| Merck | 14,6369 |
| BRN | 1909299 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 92-24-0(CAS DataBase Reference) |
| EPA Substance Registry System | Naphthacene (92-24-0) |
Safety information for NAPHTHACENE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for NAPHTHACENE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![BENZO[B]NAPHTHACENE](https://img.chemicalbook.in/CAS/20180808/GIF/135-48-8.gif)
![DIBENZO[A,J]CORONENE](https://img.chemicalbook.in/CAS/GIF/190-72-7.gif)
![DIBENZO[A,L]TETRACENE](https://img.chemicalbook.in/CAS/GIF/226-86-8.gif)
You may like
-
 Naphthacene (purified by sublimation) CAS 92-24-0View Details
Naphthacene (purified by sublimation) CAS 92-24-0View Details
92-24-0 -
 Naphthacene 95% CAS 92-24-0View Details
Naphthacene 95% CAS 92-24-0View Details
92-24-0 -
![Naphthacene [for organic electronics] CAS 92-24-0](https://img.chemicalbook.in//Content/image/CP5.jpg) Naphthacene [for organic electronics] CAS 92-24-0View Details
Naphthacene [for organic electronics] CAS 92-24-0View Details
92-24-0 -
![Benz[b]anthracene CAS 92-24-0](https://img.chemicalbook.in//Content/image/CP5.jpg) Benz[b]anthracene CAS 92-24-0View Details
Benz[b]anthracene CAS 92-24-0View Details
92-24-0 -
![Benz[b]anthracene CAS 92-24-0](https://img.chemicalbook.in//Content/image/CP5.jpg) Benz[b]anthracene CAS 92-24-0View Details
Benz[b]anthracene CAS 92-24-0View Details
92-24-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1