NADOLOL
Synonym(s):Nadolol
- CAS NO.:42200-33-9
- Empirical Formula: C17H27NO4
- Molecular Weight: 309.4
- MDL number: MFCD00079476
- EINECS: 255-706-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
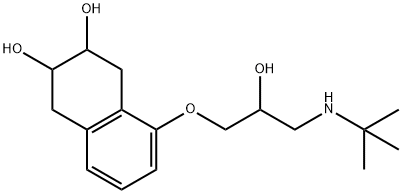
What is NADOLOL?
Absorption
Oral doses of nadolol are approximately 30% absorbed. In healthy subjects, nadolol has a Tmax of 2.7h with a Cmax or 69±15ng/mL following a 60mg oral dose and 132±27ng/mL after a 120mg oral dose. The AUC following a 60mg oral dose was 1021ng*h/mL and following a 120mg oral dose was 1913±382ng*h/mL.
Toxicity
The oral LD50 in mice is 4500mg/kg.
Patients experiencing an overdose may present with bradycardia, cardiac failure, hypotension, and bronchospasm. An overdose may be treated with atropine for bradycardia, digitalis and diuretics for cardiac failure, vasopressors for hypotension, and beta-2 stimulants for bronchospasms, as well as gastric lavage and hemodialysis.
Chemical properties
White Solid
Originator
Solgol,Heyden,W. Germany,1978
The Uses of NADOLOL
Labelled Nadolol (N201052). β-Adrenergic
The Uses of NADOLOL
CNS stimulant
The Uses of NADOLOL
Labeled Nadolol, intended for use as an internal standard for the quantification of Nadolol by GC- or LC-mass spectrometry.
Indications
Nadolol is indicated to treat angina pectoris and hypertension. Another product formulated with bendroflumethiazide is indicated to treat hypertension.
Background
Nadolol is a nonselective beta adrenal receptor blocker that is used to lower blood pressure. Nonselective beta adrenal receptor blockers may no longer be first line in the treatment of hypertension as newer generations of beta adrenal receptor blockers have higher selectivity and offer better rates of adverse effects.
Nadolol was granted FDA approval on 10 December 1979.
What are the applications of Application
Nadolol is a β-adrenergic blocker
Manufacturing Process
(a) cis-5,6,7,8-Tetrahydro-1,6,7-naphthalenetriol: A solution of 29.2 g (0.2
mol) of 5,8-dihydro-1-naphthol and 40 ml of acetic anhydride in 100 ml of
pyridine is prepared. After 16 hours the solvent is removed in vacuo and the
residue dissolved in ether and washed with 200 ml of 5% hydrochloric acid,
water, 200 ml of 10% sodium hydroxide, saturated salt solution and dried.
Solvent removal gives 34.2 g (90.5%) of crude acetate which is dissolved in
900 ml of acetic acid and 36 ml of water. 53.3 g (0.32 mol) of silver acetate is
added followed by 40.6 g (0.16 g-atom) of iodine. The slurry is heated with
good stirring at 85°10°C for 3 hours under nitrogen, cooled and filtered. The
filtrate is evaporated in vacuo and the residue dissolved in 250 ml of methanol
and cooled to 0°C.
A solution of 40 g of sodium hydroxide in 200 ml of water is added under
nitrogen and the mixture stirred overnight. The bulk of the methanol is
removed in vacuo whereupon a solid forms. The solid is separated by
filtration, dissolved in 150 ml of water and acidified with 20 ml of
concentrated hydrochloric acid. Cooling gives a solid which is filtered and dried
to give 16.5 g cis-5,6,7,8-tetrahydro-1,6,7-naphthalenetriol, melting point 184.5°C to 187°C. Three recrystallizations from absolute ethanol give the
analytical sample, melting point 188°C to 188.5°C.
(b) 2,3-cis-1,2,3,4-Tetrahydro-5-[2,3-(epoxy)-propoxy]-2,3-naphthalenediol:
A solution of 1.20 g (0.03 mol) of sodium methoxide and 5.4 g (0.03 mol) of
cis-5,6,7,8-tetrahydro-1,6,7-naphthalenetriol in 200 ml of methanol is
prepared under nitrogen. The residue obtained upon solvent removal is stirred
overnight with 200 ml of dimethylsulfoxide and 4.65 g (0.05 mol) of
epichlorohydrin under nitrogen. The bulk of the solvent is removed at 50°C at
0.1 mm and the residue dissolved in 100 ml of water. Extraction with
chloroform (10 x 200 ml) gives a solid which is recrystallized from 150 ml of
hexane-ethyl acetate to give epoxy diol of the above title.
(c)2,3-cis-1,2,3,4-Tetrahydro-5-[2-hydroxy-3-(tert-butylamino)propoxy]-2,3-
naphthalenediol: A mixture of 2,3-cis-1,2,3,4-tetrahydro-5-[2,3-
(epoxy)propoxy]-2,3-naphthalenediol (melting point 104°C to 107°C, one spot
on TLC-alumina, 5% methanol in chloroform, iodine visualization) and 22 ml
of tert-butylamine is heated at 85°C to 95°C for 15 hours in a Parr bomb and
the excess amine removed in vacuo. The solid obtained by trituration of the
residue with ether is filtered and recrystallized from benzene to give 3.4 g,
melting point 124°C to 136°C.
brand name
Corgard (King).
Therapeutic Function
Antiarrhythmic
Pharmacokinetics
Nadolol is a nonselective beta adrenal receptor blocker that is used to lower blood pressure. It has a long duration of action as it is usually taken once daily and a wide therapeutic index as patients start at doses of 40mg daily but may be increased to doses as high as 240mg daily. Patients taking nadolol should not aburptly stop taking it as this may lead to exacerbation of ischemic heart disease.
Clinical Use
Beta-adrenoceptor blocker:
Hypertension
Angina
Arrhythmias
Migraine
Thyrotoxicosis
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect. Analgesics: NSAIDs antagonise hypotensive effect.
Anti-arrhythmics: increased risk of myocardial
depression and bradycardia; increased risk of
bradycardia, myocardial depression and AV block
with amiodarone; increased risk of myocardial
depression and bradycardia with flecainide.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Antihypertensives: enhanced hypotensive effect;
increased risk of withdrawal hypertension with
clonidine; increased risk of first dose hypotensive
effect with post-synaptic alpha-blockers such as
prazosin.
Antimalarials: increased risk of bradycardia with
mefloquine.
Antipsychotics enhanced hypotensive effect with
phenothiazines.
Calcium-channel blockers: increased risk of
bradycardia and AV block with diltiazem;
hypotension and heart failure possible with
nifedipine and nisoldipine; asystole, severe
hypotension and heart failure with verapamil.
Cytotoxics: possible increased risk of bradycardia
with crizotinib.
Diuretics: enhanced hypotensive effect.
Fingolimod: possibly increased risk of bradycardia.
Moxisylyte: possible severe postural hypotension.
Sympathomimetics: severe hypertension with
adrenaline and noradrenaline and possibly with
dobutamine.
Metabolism
Nadolol is not metabolized by the liver in humans.
Metabolism
Nadolol (Corgard) is slowly and incompletely absorbed from the gastrointestinal tract, and only 30% of an orally administered dose is absorbed. Appreciable metabolism does not seem to occur; nadolol is excreted primarily unchanged in the urine and feces.The plasma half-life is quite long, approaching 24 hours, which permits dosing once per day.
Properties of NADOLOL
| Melting point: | 125-130°C |
| Boiling point: | 449.67°C (rough estimate) |
| Density | 1.0449 (rough estimate) |
| refractive index | 1.5420 (estimate) |
| storage temp. | Refrigerator |
| solubility | Slightly soluble in water, freely soluble in ethanol (96 per cent), practically insoluble in acetone. |
| form | neat |
| pka | 9.67(at 25℃) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 8.30g/L(25 ºC) |
| EPA Substance Registry System | 2,3-Naphthalenediol, 5-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,2,3,4-tetrahydro- (42200-33-9) |
Safety information for NADOLOL
Computed Descriptors for NADOLOL
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Nadolol 98%View Details
Nadolol 98%View Details
42200-33-9 -
 Nadolol 42200-33-9 99%View Details
Nadolol 42200-33-9 99%View Details
42200-33-9 -
 Nadolol 98%View Details
Nadolol 98%View Details
42200-33-9 -
 Nadolol CAS 42200-33-9View Details
Nadolol CAS 42200-33-9View Details
42200-33-9 -
 Nadolol CAS 42200-33-9View Details
Nadolol CAS 42200-33-9View Details
42200-33-9 -
 Nadolol CAS 42200-33-9View Details
Nadolol CAS 42200-33-9View Details
42200-33-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
